
 ���백���黥Ϊ�ȵ�������л�С������CH3CH3 ��д�ṹ��ʽ����
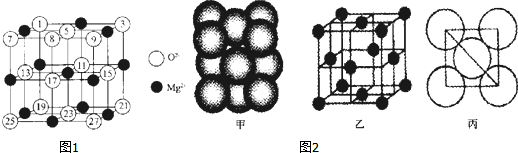
���백���黥Ϊ�ȵ�������л�С������CH3CH3 ��д�ṹ��ʽ���� ���� ��1�������Ȼ��Ƶľ����ṹ�ж���λ���������Ȼ��Ƶľ����ṹ��֪������þ��þԭ�Ӻ�þԭ�Ӵ���С�����εĶԽ����ϣ�
��2�����ݦ�=$\frac{m}{V}$�����ܶȣ�
��3�����ݵ�ԭ���ṩһ�Թ��õ��ӶԸ���ԭ���γ���λ�������ݵȵ����������ͬ�ĵ�����Ŀ��ԭ����Ŀ��������
��� �⣺��1�����Ȼ��Ƶľ����ṹ��֪�������Ӻ������ӵ���λ������6��������MgO������Mg��O����λ��Ҳ����6�����Ȼ��Ƶľ����ṹ֪������þ��þԭ�Ӻ�þԭ�Ӵ���С�����εĶԽ����ϣ�����ͼ��֪����Ӧ��Ϊ��ɫ��
�ʴ�Ϊ��6��6����ӦΪ��ɫ��
��2����ԭ�Ӱ뾶Ϊd����ͼ����֪�������ζԽ��߳���Ϊ4d����������ⳤΪ$\frac{\sqrt{2}}{2}$��4d=22$\sqrt{2}$d���������Ϊ��2$\sqrt{2}$d ��3=16$\sqrt{2}$d3 �������к���ԭ����Ŀ=8��$\frac{1}{8}$+6��$\frac{1}{2}$=4��Al���ԭ������ΪM���ʾ���������=4��$\frac{M}{N{\;}_{A}}$g���ʾ�����ܶ�=$\frac{\frac{4M}{{N}_{A}}}{16\sqrt{2}d{\;}^{3}}$=$\frac{M}{4\sqrt{2}{d}^{3}•N{\;}_{A}}$��
�ʴ�Ϊ��$\frac{M}{4\sqrt{2}{d}^{3}•N{\;}_{A}}$��
��3����ԭ���ṩһ�Թ��õ��ӶԸ���ԭ���γ���λ����������ĽṹʽΪ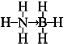 ���ȵ����������ͬ�ĵ�����Ŀ��ԭ����Ŀ���백���黥Ϊ�ȵ�������л�С������CH3CH3��
���ȵ����������ͬ�ĵ�����Ŀ��ԭ����Ŀ���백���黥Ϊ�ȵ�������л�С������CH3CH3��
�ʴ�Ϊ��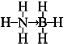 ��CH3CH3��
��CH3CH3��
���� ������Ҫ���������Ӿ��塢�������塢���Ӿ���Ľṹ�ص㼰���ʣ��ۺ��Խ�ǿ����һ�����Ѷȣ�����ʱҪע�⾧��ṹ���й�֪ʶ��������ã�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ũ���� | B�� | Ũ���� | C�� | Ũ��ˮ | D�� | ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
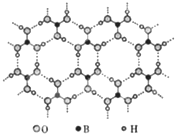 �����ᣨH3BO3����һ�ֲ�״�ṹ��ɫ���壬���ڵ�H3BO3����ͨ�������������ͼ���������й�˵��������ǣ�������
�����ᣨH3BO3����һ�ֲ�״�ṹ��ɫ���壬���ڵ�H3BO3����ͨ�������������ͼ���������й�˵��������ǣ�������| A�� | �����ᾧ�����ڷ��Ӿ��� | B�� | H3BO3���ӵ��ȶ���������й� | ||
| C�� | ��������ԭ������㲻��8e-�ṹ | D�� | ��1mol H3BO3�ľ�������3mol��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �÷�Һ�ķ�������ú�ͺ�NaCl��Һ�Ļ���� | |
| B�� | �ýᾧ���ᴿNaCl��KNO3������е�KNO3 | |
| C�� | ���������Ҵ���ˮ�Ļ���� | |
| D�� | �ü��ȷ��������Ȼ�淋Ļ�����ʾ�������������Ȼ�������ֽ⣬��ȴ�������ֶ���������ԭ���壩 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
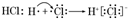
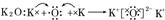
| A�� | �٢� | B�� | �� | C�� | �� | D�� | ������ȷ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ̼������Һ�ʼ��ԣ����н�ǿ��ȥ�������� | |
| B�� | Al2��SO4��3��NaHCO3��Һ���������������е����� | |
| C�� | NaCl�������������͵�ζ�� | |
| D�� | ����ʱ��NH4Cl��Һ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �屽�л����壬����KI��Һ�� | |
| B�� | �����л�����ϩ��ͨ��������һ�������·�Ӧ��ʹ��ϩת��Ϊ���� | |
| C�� | �������л���ŨHNO3��ŨH2SO4�����䵹��NaOH��Һ�������ã���Һ | |
| D�� | ��ϩ�л���SO2��CO2������ͨ������KMnO4��Һ��ϴ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.2 | B�� | 0.6 | C�� | 0.1 | D�� | 0.3 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com