

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ����С��Χ�� nm
����С��Χ�� nm ��Һ��ͭ��Ӧ�����ӷ���ʽ
��Һ��ͭ��Ӧ�����ӷ���ʽ ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 g2+��1 mol Al3+�Ļ����Һ���ְ���ͼ���������μ���7 mol NaOH��Һ��1 mol���ᣬͨ��1 mol CO2���壬������Ϊ��ϳ��������ʵ������뻭�����������淴Ӧ�ı仯���ߣ���д��������Ӧ�����ӷ���ʽ��
g2+��1 mol Al3+�Ļ����Һ���ְ���ͼ���������μ���7 mol NaOH��Һ��1 mol���ᣬͨ��1 mol CO2���壬������Ϊ��ϳ��������ʵ������뻭�����������淴Ӧ�ı仯���ߣ���д��������Ӧ�����ӷ���ʽ��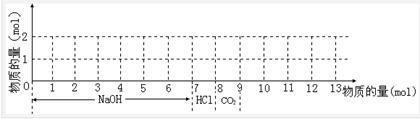
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����������Һ��ͨ������CO2��2C6H5O��+CO2+H2O��2C6H5OH+CO32�� |
| B��FeSO4��������Һ�м�H2O2��2Fe2++H2O2+2H+=2Fe3++2H2O |
| C���ð�ˮ����������SO2��NH3 ��H2O+SO2==NH4++HSO3�� |
| D��̼��������Һ�м������ʯ��ˮ��HCO3��+OH��==CO32��+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���������Ȼ�����Һ��Ӧ�� SO42��+ Ba2+ =BaSO4�� |
| B��̼��������ᷴӦ�� CaCO3 + 2H+ =Ca2+ + CO2�� + H2O |
C����Ͷ������ͭ��Һ�У� 2Na + Cu2+ = 2N a+ + Cu a+ + Cu |
| D������ͨ��ˮ�У�Cl2 + H2O = H+ + Cl��+ HClO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������ʵ���Ũ�ȵ�FeBr2��CuCl2�����Һ�ö��Ե缫������������ Cu 2++ 2Br�� 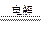 ��Cu +Br2 ��Cu +Br2 |
| B��̼����þ��Һ�м����������ռ���Һ�� Mg2+ + 2HCO3- + 2OH-= MgCO3��+ CO32- + 2H2O |
| C��Fe(NO3)3��������Һ��ͨ���������⣺2Fe3+��H2S===2Fe2+��S����2H+ |
| D��H218O��Ͷ��Na2O2��4H218O��O22��===2H2O��4OH����18O2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 �� �� ��
�� �� ��| A��������Ͷ��ˮ��K+H2O=K++OH-+H2�� |
| B���������������ᷴӦ��OH-+H+=H2O |
| C����ʳ�׳�ˮ��CaCO3+2H+=Ca2++CO2��+H2O |
D����ⱥ��ʳ��ˮ2Cl-+2H2O H2��+Cl2��+2OH- H2��+Cl2��+2OH- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A���ö��Ե缫����Ȼ�þ��Һ��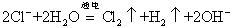 |
| B�����Ȼ�������Һ��ϡ�����ϣ�3Fe2����NO3����4H��=3Fe3����NO����2H2O |
| C��̼�������Һ����������������Һ��Ӧ�� + OH��NH3��H2O |
| D��NaAlO2��Һ��ͨ�����CO2��2AlO2�� + CO2 + 3H2O ="==" 2Al(OH)3�� + CO32�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ƫ��������Һ������ | B������������� Һ�ͳ����ʯ��ˮ Һ�ͳ����ʯ��ˮ |
| C����������Һ�Ͷ�����̼ | D����������Һ�Ͱ�ˮ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com