
����Ŀ����1�������£���A��B��C��D������ɫ��Һ�����Ƿֱ���CH3COONa��Һ��NH4Cl��Һ�������NaNO3��Һ�е�һ�֡���֪A��B��ˮ��Һ��ˮ�ĵ���̶���ͬ��A��C��Һ��pH��ͬ����B��________��C��________��
��2��ʵ��������FeSO4��Һ���ܽ�ʱ��Ҫ����������ϡ���ᣬ��ԭ����__________________________��������Ϻ�Ҫ����������м����Ŀ����__________________________����AlCl3��Һ�������գ����õ�����Ҫ���������___________��AlCl3��Һ�����Ե�ԭ���ǣ������ӷ���ʽ˵����__________________��
���𰸡� CH3COONa ���� Ϊ������Fe2+��ˮ�� ��ֹFe2+�������е�����������Fe3+ Al2O3 Al3++3H2O![]() Al(OH)3+3H+
Al(OH)3+3H+
�������������������1��CH3COONa��ǿ�������Σ�ˮ��ʼ��ԣ�NH4Cl��ǿ�������Σ�ˮ������ԣ�������Һ�����ԣ�NaNO3��ǿ��ǿ��������Һ�����ԣ���2������������Һ�У�������������Һ�з���ˮ�����������������������ӣ������������ױ������������ӣ�Al3+����ˮ�⣬ˮ��ķ���ʽΪAl3++3H2O![]() Al(OH)3+3H+��ˮ�����Һ�����ԣ����ɺ����չ����У�HCl�ӷ���Al(OH)3���ȶ�������ʱ�ֽ�����Al2O3��
Al(OH)3+3H+��ˮ�����Һ�����ԣ����ɺ����չ����У�HCl�ӷ���Al(OH)3���ȶ�������ʱ�ֽ�����Al2O3��
��������1��CH3COONa��Һ��NH4Cl��Һ�������NaNO3������Һ��CH3COONaΪǿ�������Σ�ˮ��ʼ��ԣ��ٽ�ˮ�ĵ��룬NH4Cl��ҺΪǿ�������Σ�ˮ������ԣ��ٽ�ˮ�ĵ��룬������Һ�����ԣ�����ˮ�ĵ��룬NaNO3Ϊǿ��ǿ���Σ���Һ�����ԣ�A��C��Һ��pH��ͬ��A��CΪNH4Cl��Һ�����A��B��Һ��ˮ�ĵ���̶���ͬ��A��BΪCH3COONa��Һ��NH4Cl��Һ����AΪNH4Cl��Һ��BΪCH3COONa��Һ��CΪ���ᣬDΪNaNO3��Һ��
��2��ʵ�������Ƶ�FeSO4��Һʱ�������������Ӳ���ˮ��������������������Ӧ�����ӷ���ʽΪ��Fe2++2H2O![]() Fe��OH��2+2H+��ͨ������ϡ���ᣬʹ������Ũ������ˮ��ƽ�������ƶ���������Fe2+ˮ�⣻����Fe2+���ױ�O2��������Ϊ��ɫ��Fe3+������������Ϻ�Ҫ����������м����ֹFe2+�������е�����������Fe3+���Ȼ���Ϊǿ�������Σ�Al3+����ˮ�⣬ˮ��ķ���ʽΪAl3++3H2O
Fe��OH��2+2H+��ͨ������ϡ���ᣬʹ������Ũ������ˮ��ƽ�������ƶ���������Fe2+ˮ�⣻����Fe2+���ױ�O2��������Ϊ��ɫ��Fe3+������������Ϻ�Ҫ����������м����ֹFe2+�������е�����������Fe3+���Ȼ���Ϊǿ�������Σ�Al3+����ˮ�⣬ˮ��ķ���ʽΪAl3++3H2O![]() Al(OH)3+3H+��ˮ�����Һ�����ԣ����ɺ����չ����У�HCl�ӷ���ˮ��ƽ�ⲻ�������ƶ�����ȫ������������������Al(OH)3���ȶ�������ʱ�ֽ�����Al2O3��
Al(OH)3+3H+��ˮ�����Һ�����ԣ����ɺ����չ����У�HCl�ӷ���ˮ��ƽ�ⲻ�������ƶ�����ȫ������������������Al(OH)3���ȶ�������ʱ�ֽ�����Al2O3��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ�Ǵ���ʿ����Ҫ�ɷ�ΪAl2O3 �� ����������SiO2��Fe2O3�����ʣ�����ȡAl2O3������AlN�Ĺ������̣� 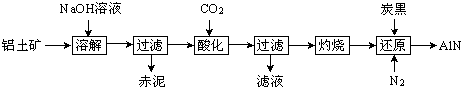
��1�����ܽ⡱ʱ����Һ�еĹ�������ƫ�����Ʒ�����Ӧ��2Na2SiO3+2NaAlO2+2H2O�TNa2Al2Si2O8��+4NaOH �������Ҫ�ɷ�Ϊ��д����ѧʽ����
��2�����ữ��ʱͨ�����CO2��NaAlO2��Ӧ����Al��OH��3 �� ��Һ����Ҫ�ɷ�Ϊ��д����ѧʽ����ʵ���ҹ������õ��IJ����������ձ�������������
��3������ԭ��ʱ��̿���ڸ����±�����ΪCO����Ӧ�Ļ�ѧ����ʽΪ ��
��4����ȡ���ݲ�ͬ�����ĵ�������Ʒ����������ֻ����̿�ڣ��ֱ�ӵ�20.00mL��ͬŨ�ȵ�NaOH��Һ�У���ַ�Ӧ���ʵ�����������ʾ�� ����֪��AlN+NaOH+H2O�TNaAlO2+NH3����
ʵ����� | �� | �� | �� |
���뵪������Ʒ������/g | 4.1 | 8.2 | 12.3 |
���ɰ��������/L����״���� | 1.456 | 2.912 | 4.256 |
�ٸ���Ʒ��AlN����������Ϊ���٣���д��������̣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ܱ������У���ӦX2��g����Y2��g��![]() 2XY��g�� ��H<0���ﵽ��ƽ�⡣�ڽ��ı�ijһ�����ﵽ��ƽ�⣬�Դ˹��̵ķ�����ȷ����( )
2XY��g�� ��H<0���ﵽ��ƽ�⡣�ڽ��ı�ijһ�����ﵽ��ƽ�⣬�Դ˹��̵ķ�����ȷ����( )
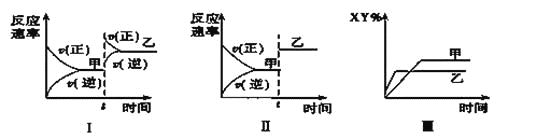
A. ͼ��������ѹǿ�ı仯���
B. ͼ����һ���Ǽ�������ı仯���
C. ͼ��������ѹǿ�������¶ȵı仯���
D. ͼ��һ���������¶ȵı仯���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ħ����[��NH4��2Fe��SO4��26H2O]�Ƿ�����ѧ�е���Ҫ�Լ���Ħ���θ����������ȿ���ȫ�ֽ����ɺ���ɫ������������ijѧϰС����̽����ֽ���
��1������ͬѧ�������ͼ1��ʾʵ��װ�ã� 
��i��ʵ���У�װ��B�й۲쵽�������� ��
��ii����ʯ�ҵ���Ҫ������ ��
��iii��װ��D�й۲쵽��̪��Һ���ɫ���ɴ˿�֪Ħ���ηֽ�IJ���������д��ѧʽ����
��iv�����ʵ����֤װ��A�й�������ﲻ��FeO������Ҫ˵��ʵ�����������ͽ��ۣ���
��2������ͬѧ��ΪĦ�������ȷֽ�IJ����л�����SO2��SO3��N2 �� Ϊ������֤���ü���ʵ���е�װ��A��������ͼ2��ʾװ�ý���ʵ�飮
˵���˹�����ﲻ��FeO|����
��i������ͬѧ��ʵ��װ���У��������ӵĺ���˳��ΪA�� �� װ��G�������������
��ii��ʵ���У�ȷ����Ħ����7.8400g����ּ��ȷ�Ӧ���װ��A�й�������������Ϊ1.6000g��װ��G�����ɰ�ɫ����������Ϊ3.4950g��װ��H���ռ���112mL N2����״���£���д��Ħ�������ȷֽ�Ļ�ѧ����ʽ�� �� ���ֽ���ﱻ������գ�����������ʧ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2015��8��12�գ�������������ը�¹�ȷ�����軯����NaCN�����������Ƶȣ��軯�ƶ��Ժ�ǿ����ˮ���������ж���ȼ�軯����HCN�����塣�軯��ķе�ֻ��26 ��������൱���ӷ������������ʹ���������ж��ķ��ա�ͬʱ�軯�������������ƻᷢ����ը���ش���������:
��1��д���軯����ˮ�����軯����������ӷ���ʽ_________________________
��2����ը�ֳ�Լ700�ֵ��軯�ƴ�Լ��Ҫ900�ֵ�˫��ˮ���������軯����˫��ˮ�������ͷų�����ͬʱ������ɫ���壬ʹ���軯�ƵĶ��Դ�ͣ�д���軯����˫��ˮ��Ӧ�Ļ�ѧ����ʽ________________________________��
��3����ը�����ڷ�ˮ�е�CN��������Cr2O![]() �������ⶨ�������̽��з�ˮ������
�������ⶨ�������̽��з�ˮ������
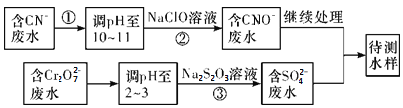
������������ˮ��������Ҫʹ�õķ�����____________
a����������������b���кͷ���������c����������������d��������ԭ��
�ڢ��з�Ӧ��������ų����÷�Ӧ�����ӷ���ʽΪ_________________��
�۲�����У�ÿ����0.4 mol Cr2O![]() ʱת�Ƶ���2.4 mol���÷�Ӧ�����ӷ���ʽ_________��
ʱת�Ƶ���2.4 mol���÷�Ӧ�����ӷ���ʽ_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��CH2OH��CHOH��4CHO�������ǵĽṹ��ʽ���������ܷ����Ļ�ѧ��Ӧ�ǣ� ��
A.ȼ��
B.������Ӧ
C.ˮ�ⷴӦ
D.�����Ʊ���Cu��OH��2��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ڹ�����ռ����Ҫ��λ��
I.�ϳɰ���ҵ�У���ʼʱ���������������ױ�Ϊ1��3ʱ��ÿ��Ӧ1mol N2���ų�92.2kJ��������ͼΪ�ϳɰ���Ӧ�ڲ�ͬ�¶Ⱥ�ѹǿ��ʹ����ͬ���������£�ƽ�������а������������
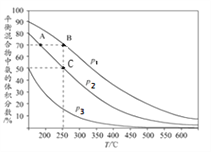
����ͼ��֪��p1��p2��p3�Ĵ�С��ϵΪ________,������_____________________________
��A��B�����ɰ��������ʴ�С��ϵΪ_____________��C�㣬N2��ת����Ϊ_____��
�����й��ںϳɰ���˵����ȷ��_________ (�����)��
A.�Ͽ�1��N��N����ͬʱ��6��N��H���γɣ���Ӧһ���ﵽƽ��״̬
B.��������ƽ����Է����������ٸı�״̬����Ӧһ���ﵽƽ��״̬
C.���ڡ�H<0����S>0���ʺϳɰ���Ӧһ�����Է�����
D.����n(N2):n(H2)�ı�ֵ������������H2��ת����
II.�������Simons�ȿ�ѧ�ҷ����˲���ʹ�����ѻ�Ϊ��Ϳ�ֱ������ȼ�ϵ�صķ��������ط�ӦΪ4NH3+3O2==2N2+6H2O��д�������ĵ缫��Ӧʽ��__________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������У���ʹ���Ը��������Һ��ɫ�������ܷ�����ѧ��Ӧʹ��ˮ��ɫ���ǣ� ��
A.��ϩ
B.��
C.�ױ�
D.����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com