
������Һ���������ʵ���Ũ�ȹ�ϵ��ȷ����
| A��25��ʱ��pH=7��NH4Cl��NH3?H2O�����Һ��c��H+��=c��OH-��=c��NH4+��=c��Cl-�� |
| B��0.1mol/LNa2S��Һ��c��OH-��=c��H+��+c��HS-��+c��H2S�� |
| C��25��ʱ��pH=2��CH3COOH��pH=12��NaOH�������ϣ� c��CH3COO-��+c��H+����c��Na+��+c��OH-�� |
| D��0.1mol/LNa2CO3��Һ��2c��CO32-��+2c��HCO-��+2c��H2CO3��=c��Na+�� |
D
�������������A��������Һ����c��H+��=c��OH-�����ַ��ϵ���غ㣬c��H+��+ c��NH4+��= c��OH-��+ c��Cl-����������c��NH4+��=c��Cl-��> c��H+��=c��OH-��,����B��0.1mol/LNa2S��Һ�У����������غ��c��OH-��=c��H+��+c��HS-��+2c��H2S��������C��25��ʱ��pH=2��CH3COOH��pH=12��NaOH��Һ�У�c(CH3COOH)>c(NaOH),�������Ϻ����ҺʵΪCH3COOH��CH3COONa����c��CH3COO-��+c��H+��>c��Na+��+c��OH-��,����D������Ԫ���غ��2c��CO32- ��+2c��HCO- ��+2c��H2CO3��=��Na+������ȷ����ѡD��
���㣺������Һ������Ũ�ȵĴ�С��ϵ���غ���ɵ�Ӧ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��֪����������K2SO4��MgSO4��2CaSO4��ˮ�д�������ƽ�⣺
K2SO4��MgSO4��2CaSO4(s)=2Ca2++2K++Mg2++4SO42-����ͬ�¶��£�K+�Ľ���Ũ�����ܽ�ʱ��Ĺ�ϵ����ͼ��ʾ��������˵���������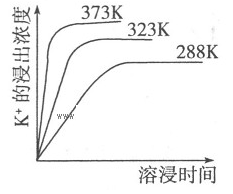
| A�������ϵ�м��뱥��NaOH��Һ���ܽ�ƽ�������ƶ� |
| B�������ϵ�м��뱥��̼������Һ���ܽ�ƽ�������ƶ� |
| C����ƽ���Ksp=c(Ca2+)��c(K+)��c(Mg2+)��c(SO42-) |
| D�������¶ȣ���Ӧ��������ƽ��������Ӧ�����ƶ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��Ca��OH��2��������ˮ�У�һ��ʱ���ﵽ����ƽ�⣺

 ������˵����ȷ���ǣ�������
������˵����ȷ���ǣ�������
| A������������Һ�м�CaO����Һ��pH���� |
| B������Һ���ȣ���Һ��pH���� |
| C������Һ�м���Na2CO3��Һ�����й����������� |
| D������Һ�м�������NaOH���壬Ca��OH��2������������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
ij��ѧ�о���ѧϰС��Ե������Һ�����µĹ����ܽᣨ���ڳ����£���������ȷ����
��pH=l��ǿ����Һ��ˮϡ�ͺ���Һ�и�����Ũ��һ����С
��pH=2�������pH=l�����ᣬc��H����֮��Ϊ2:1
��pH��ȵ�������Һ��a��CH3 COONa��b��C6H5 ONa��c��NaHCO3��d��NaOH������Һ���ʵ���Ũ����С�����˳��Ϊd<b<c<a
��NH4HSO4��Һ�еμ�NaOH��Һ����ҺpH=7����c��Na����=2c��SO42����
����֪�������ƽ�ⳣ��ΪKa�������ˮ�ⳣ��ΪKh��ˮ�����ӻ�ΪKw�������߹�ϵΪKa��Kh=Kw
�ס�������Һ����ǿ�������Һ����֪����Һ��pH������ҺpH����������ס�������Һ�������ϣ����ҺpH���ܵ���7
| A���ۢݢ� | B���ۢܢ� | C���ܢݢ� | D���٢ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����������ȷ���� (����)
| A���к͵�����������ʵ���Ũ�ȵ�����ʹ�����Һ�����������������ƶ��ڴ��� |
| B��������������Һ�Ͱ�ˮ��ϡ��һ�������ߵ�c(OH��)�����ٵ�ԭ����һ�� |
| C�������£�ij��Һ����ˮ�������c(OH��)��1��10��10mol/L������Һ���������� |
| D�������������ʵ���Ũ���Ǵ�����������������c(H��)Ҳ�Ǵ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����£�����0.10 mol��L��1�İ�ˮ�������ж���ȷ���ǣ�������
| A����AlCl3��Һ������Ӧ�����ӷ���ʽΪAl3����3OH��=Al��OH��3�� |
| B����ˮϡ�ͺ���Һ��c��NH4+����c��OH������� |
| C����HNO3��Һ��ȫ�кͺ���Һ�������� |
| D������Һ��pH��13 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
Ϊ̽��ij���ε�ˮ�������ȷ�Ӧ������λͬѧ�ֱ����������ʵ�鷽����
| ͬѧ | ʵ����� |
| �� | ������茶�������ˮ����ˮ���½���˵�������ˮ�������ȵ� |
| �� | ������ʹ��Һ�е�Fe3��ת����Fe(OH)3������˵��Fe3��ˮ�������ȵ� |
| �� | ͨ��ʵ�鷢��ͬŨ�ȵ��ȵĴ�����Һ����Ĵ�����Һȥ����Ч���ã�˵��̼����ˮ�������ȵ� |
| �� | �ڴ�������Һ�е����̪��Һ������(������ˮ����)������ɫ���˵��������ˮ�������ȵ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����£�0.1 mol��L��1��HA��Һ��c(OH��)/c(H��)��1��10��8��������������ȷ����(����)
A��0.01 mol��L��1HA����Һ��c(H��)��1��10��4mol��L��1
B��pH��3��HA��Һ��pH��11��NaOH��Һ�������Ϻ�������Һ��c(Na��)��c(A��)��c(OH��)��c(H��)
C��Ũ�Ⱦ�Ϊ0.1 mol��L��1��HA��Һ��NaA��Һ�������Ϻ�������Һ�����ԣ���c(OH��)��c(H��)��c(HA)��c(A��)
D��pH��3��HA��Һ��pH��11��NaOH��Һ�������1?10��Ϻ�������Һ��c(OH��)��c(A��)��c(H��)��c(Na��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����£���0.100 mol/L NaOH ��Һ�ֱ�ζ�20.00 mL 0.100 mol/L������ʹ��ᣬ�ζ���������ͼ��ʾ������˵����ȷ���ǣ� ��
| A����ֱ��ʾ����ʹ���ĵζ����� |
B��V(NaOH)��10.00 mL ʱ�� ��1 ��1 |
| C��pH��7ʱ������������NaOH��Һ�������� |
| D��V(NaOH)��20 .00 mL ʱ��c(Cl��)��c(CH3COO��) |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com