
ij�о���ѧϰС��Ի�ԭ������ˮ�����ķ�Ӧ������п�ѧ̽������֪Ca(OH)2�ķֽ��¶�Ϊ580�棬������ˮ������Ӧ���¶�Ϊ900�棺������ͼ��ʾʵ��װ�ã������˻�ԭ������ˮ�����ķ�Ӧʵ�飬ʵ���й۲쵽����Һ�в����˴��������ݡ�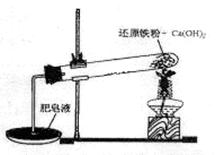
��1��ʵ����Ca(OH)2�������� ��ʵ���в�������������� ��
��2��Ϊ��һ��̽����ԭ������ˮ������Ӧ�������ijɷ֣��о���ѧϰС�齫��Ӧ��Ĺ��徭������õ���ɫ��������壬��Ժ�ɫ��������壬��С��������µļ��貢��������ص�ʵ�飺
����һ������ΪFeO
�����������ΪFe3O4
��������
����ѡ�������Լ������ᡢKSCN��Һ��K3Fe(CN)6 ��Һ����ˮ��֤������һ������
| ���� | ���� | ���� |
| | | ����һ������ |

��1���ṩ��Ӧ�����ˮ���� H2
��2������ΪFe3O4��FeO���� ���� ���� ȡ�����������Թ��У�������������ܽ⣬�ټ���KSCN��Һ���� ��Һ���ֺ�ɫ
2Fe2++Cl2=2Fe3++2Cl- ȡ���һ��ϴ��Һ���Թ��У�����ϡ�����ữ���ټ���AgNO3��Һ����û�л��ǣ���ϴ�Ӹɾ��� ���� 6.
���������������1��Fe��ˮ������ӦӦ�õ����������������H2����ˣ���Ӧ��ˮ����������580�����ϸ��·ֽ�Ca(OH)2�õ�.��2����Ϊ�������Ǻ�ɫ���������Ǻ�ɫ���ʲ������ΪFeO��Fe3O4����ߵĻ���������������ٵ�ʵ��֤�����������+3����ʱ������һ��������ʵ�������ȡ�����������Թ��У�������������ܽ⣬�ټ���KSCN��Һ������Һ���ֺ�ɫ���ڹ�������������ܽ������ˮ�ɽ�Fe2+����ΪFe3+(2Fe2++Cl2=2Fe3++2Cl-)�����ɫ����Fe(OH)3������������NaCl����ˮϴ��2~3�Σ�ȡ���һ��ϴ��Һ���Թ��У�����ϡ�����ữ���ټ���AgNO3��Һ����û�л��ǣ���ϴ�Ӹɾ����������յ�������������
��Fe3O4~ 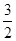 Fe2O3��5.8g Fe3O4����6g Fe2O3��
Fe2O3��5.8g Fe3O4����6g Fe2O3��
���㣺���������ӵ�����ʵ�鷽����������ۡ�


 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��9�֣�����ֲ��纣���������к��зḻ�ĵ�Ԫ�أ���Ԫ���Ե����ӵ���ʽ���ڡ�ʵ������Ӻ�������ȡ����������£�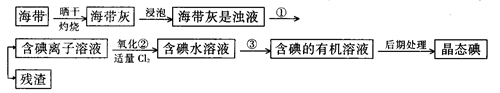
��1��ָ����ȡ��Ĺ������йص�ʵ��������ƣ�
�� ����______________________��
д��ʵ������йط�Ӧ�����ӷ���ʽ _______________________ ��
��2����ȡ��Ĺ����У��ɹ�ѡ����й��Լ���___________��
| A���ױ����ƾ� | B�����Ȼ�̼���� |
| C�����ͣ����� | D�����ͣ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
4,7-�����㶹�أ��۵㣺132.6�棩��һ����Ҫ�����ϣ��㷺�ֲ���ֲ�����,�ɼ�ױ���Ϊԭ�ϵĺϳɷ�Ӧ���£�
ʵ��װ��ͼ���£�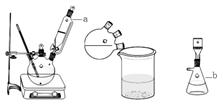
��Ҫʵ�鲽�裺
����1.��������ƿ�м���60mLŨ���ᣬ����ȴ��0�����£������µ����ױ���30mL(0.29mol)��������������26.4mL (0.21mol)�Ļ���
����2.������10���£�����12h����Ӧ��ȫ���䵹���ˮ������У�Ȼ����ˡ�ˮϴ�ô�Ʒ
����3.��Ʒ���Ҵ��ܽⲢ�ؽᾧ���ð�ɫ��״���岢��ɣ��Ƶò�Ʒ����Ϊ33.0g��
��1��ͼ����Ʒ���ƣ�a ��b ��
��2��ŨH2SO4��Ҫ��ȴ��0�����µ�ԭ���� ��
��3����Ӧ��Ҫ����12h����ԭ���� ��
��4��ȷ�����ղ�Ʒ��4,7-�����㶹�ص�ʵ����� ��
��5������ʵ�����Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijͬѧ��̽��ʳƷ���Ӽ������NH4Al(SO4)2��12H2O���·ֽ�������
��1��Ԥ�������й�����������Ԥ�ⲻ�������� ��
A��NH3��N2��SO2��H2O B��NH3��SO3��H2O
C��NH3��SO2��H2O D��NH3��N2��SO3��SO2��H2O
��2�����Լ��飺ȡһ������������������ʵ��̽�����
�ٰ�ͼʾ��װ���������ȼ������װ�õ������ԣ������� ��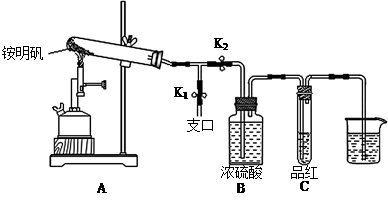
�ڼ�סֹˮ��K1����ֹˮ��K2���þƾ���Ƴ�����ա�ʵ������У�װ��A�͵�����δ������ɫ���壻�Թ�C�е�Ʒ����Һ��ɫ����֧�ڴ��ɼ��鵽NH3�������� ����װ��A��B֮���T�͵����г��ְ�ɫ���壬�ð�ɫ��������� ������һ�����ʵĻ�ѧʽ����
�۷����ó�װ��A�Թ��в����İ�ɫ���������������д��������NaOH��Һ�����ӷ���ʽ ��
��Ϊ�˷�ֹ������ʵ�����ʱ������ ������ĸ��ţ���Ȼ��Ϩ��ƾ���ơ�
A��ȡ���ձ��еĵ��� B����ֹˮ��K1 C���ر�ֹˮ��K2
��3�������ͽ��ۣ�ʵ��֤����������ǣ�1��D�е�5�����塣��ͬ�����²������N2��SO2��������Ƕ�ֵ��V��N2����V��SO2��= ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�屽��һ�ֻ���ԭ�ϣ�ʵ���Һϳ��屽��װ��ʾ��ͼ���й��������£�
| | �� | �� | �屽 |
| �ܶ�/(g��cm��3) | 0.88 | 3.10 | 1.50 |
| �е�/�� | 80 | 59 | 156 |
| ˮ���ܽ�� | �� | �� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
���嶡������ ���������������Է�ȩ��֬�ȡ�ʵ�����Ա��ӡ��嶡����[(CH3)3CCl]��Ϊԭ���Ʊ����嶡�����ӵ�ʵ�鲽�����£�
���������������Է�ȩ��֬�ȡ�ʵ�����Ա��ӡ��嶡����[(CH3)3CCl]��Ϊԭ���Ʊ����嶡�����ӵ�ʵ�鲽�����£�
����1����ͼ16��װ��������X�м���2.2 mL�嶡����(����)��1.41 g���ӣ�����ʹ������ȫ�ܽ⡣
����2����X�м�����ˮAlCl3���������������Ͻ��裬������ų���
����3����Ӧ���ͺ���X�м���8 mLˮ��1 mLŨ���ᣬ���а�ɫ����������
����4�����˵õ���ɫ���壬ϴ�ӣ���ʯ�����ؽᾧ���ö��嶡������1.8 g��
��1������X������Ϊ ��
��2������2�з�����Ҫ��Ӧ�Ļ�ѧ����ʽΪ �����÷�Ӧ���ڼ��ң��ɲ�ȡ��һ�ִ�ʩΪ ��
��3��ͼ16�е���©���������� ��
��4��ʵ������Բ�Ʒ���й�����������¡��������ں��������ͼ�� (����ĸ)��
��5����ʵ���У����嶡�����ӵIJ���Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������������C9H10O2������Ϊ��Ϣ��������������һ����ɫ��Һ�壬������ˮ������ˮ����ζ������������ˮ�㾫�����쾫�ͣ���������ʳƷ��ҵ�У�Ҳ�������л��ϳ��м��壬�ܼ��ȡ����Ʊ�����Ϊ��
��֪��
| ���� | ��Է������� | ��ɫ��״̬ | �е�(��) | �ܶ�(g��cm-3) |
| ������* | 122 | ��ɫƬ״���� | 249 | 1.2659 |
| ���������� | 150 | ��ɫ����Һ�� | 212.6 | 1.05 |
| �Ҵ� | 46 | ��ɫ����Һ�� | 78.3 | 0.7893 |
| ������ | 84 | ��ɫ����Һ�� | 80.8 | 0.7318 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
���ᣨH2C2O4����һ����Ҫ���л�����ԭ�ϡ�Ϊ̽���������ȡ�Ͳ�������ʣ���������ʵ�顣
ʵ���ʵ������������������ˮ��Һ���Ʊ����ᣬװ������ͼ��ʾ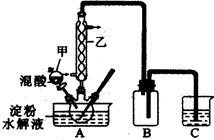
��һ�����ĵ���ˮ��Һ����������ƿ��
�ڿ��Ʒ�Ӧ�¶�55��60�棬�߽�������μ�һ�����Ļ���ᣨ65����HNO3��98����H2SO4��������2�U1.25��
�۷�Ӧ3Сʱ����ȴ�����˺����ؽᾧ�õ����ᾧ��
������������ˮ��Һ�ķ�ӦΪ��
C6H12O6��12HNO3 3H2C2O4��9NO2����3NO����9H2O
3H2C2O4��9NO2����3NO����9H2O
��1����ͼʵ��װ���������ҵ�����Ϊ��________________________��װ��B�������� ��
��2����������Ƿ�ˮ����ȫ�ķ�����______________________________________________��ʵ���̽�����������Ը�����صķ�Ӧ
��3���������Һ����μ��������ữ�ĸ��������Һʱ���ɹ۲쵽��Һ���Ϻ�ɫ��Ϊ������ɫ��д��������Ӧ�����ӷ���ʽ��____________________________________________________��
��4��ѧϰС���ͬѧ���֣����������Һ����μ��������ữ�ĸ��������Һʱ����Һ��ɫ����������졣Ϊ̽����ԭ��ͬѧ���������¶Ա�ʵ�飻
�ɴ�����Ϊ��Һ��ɫ������������ԭ����_________________________________________��
��5�����������ڹ�ҵ������Ҫ���ã���������Ʊ������������������£�
��ȡFeSO4��7H2O ������С�ձ��У�����ˮ������ϡH2SO4��Һ�ữ�������ܽ⡣�����Һ�м���һ������H2C2O4��Һ���������Һ�������У����Ͻ��裬���Ⱪ�У����л�ɫ�����������������á��������Һ���ټ�������ˮ�������ȣ����ˣ����ϴ�ӳ��������ˣ��ñ�ͪϴ�ӹ������β����ɡ�
�����ɵIJ�����������ϴ�ӳ����������Ƿ�ϴ����ȫ�ķ����� ��
���ñ�ͪϴ�ӹ������ε�Ŀ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij�о���ѧϰС����ʵ��ʱżȻ���֣������ȥ����Ĥ��þƬ����NaHCO3��Һ��Ӧ������������Ͱ�ɫ�������С��ͬѧͨ������ʵ�飬��֤���ﲢ̽����Ӧԭ����
ʵ��٣���ɰֽ��ȥþ����������Ĥ���������ʢ�������з�̪��Һ�ı���̼��������Һ���ձ��У�Ѹ�ٷ�Ӧ�������������ݺͰ�ɫ�������Һ��dz��ɫ���
����ʵ��
��1������ļ���
ʵ��ڣ���ʵ������ռ����������ȼ���������尲��ȼ�գ�����ʵ���ɫ��������Ϊ ��
��2����С��ͬѧ�Է�Ӧ�в����İ�ɫ�������������²²⣺
�²�1����ɫ���������Ϊ����������
�²�2����ɫ���������ΪMgCO3
�²�3����ɫ���������Ϊ��ʽ̼��þ[yMg(OH)2?xMgCO3]
�����һ��ʵ�����������Ƿ���MgCO3��д��ʵ�����������ͽ��ۣ� ��
��3��ʵ��ۣ�ȡʵ����е���Һ�������м�������CaCl2ϡ��Һ��������ɫ��������Һ��ɫ��dz��˵����Һ�д���CO32�����ӡ�
����ʵ��
��4��Ϊ��һ��ȷ��ʵ��I�İ�ɫ������ijɷ֣��������¶�ʵ�飬װ����ͼ��ʾ��
��ȡ��������İ�ɫ������ 7.36 g����ּ��������ٲ�������Ϊֹ����ʹ�ֽ����������ȫ������װ��A��B�С�ʵ���װ��A����0.72g��װ��B����2.64 g����ɫ������Ļ�ѧʽΪ ��
��5��д��þ�뱥��̼��������Һ��Ӧ�Ļ�ѧ����ʽ ��
��Ӧԭ������
��6��NaHCO3��Һ�д������µ���ƽ�⣺H2O H+ + OH����HCO3��
H+ + OH����HCO3�� H+ +CO32�������ƽ���ƶ��Ƕȷ���ʵ��ٲ�����������Ͱ�ɫ�������ԭ�� ��
H+ +CO32�������ƽ���ƶ��Ƕȷ���ʵ��ٲ�����������Ͱ�ɫ�������ԭ�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com