
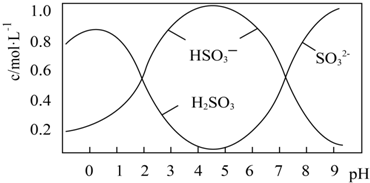
���� ��1��������ԭ��Ӧ��ת�Ƶ�����ȣ���ϵ����غ���㣻
��2������ҺpH=4.5����Һ�����ԣ���ͼ��֪����������HSO3-����ҺpHС��1����Һ��H2SO3��Ũ�ȱ�С�������������ᱻ������ֽ��йأ�Ksp[BaSO4]=c��Ba2+��•c��SO42-�����ɼ������Ҫc��Ba2+���������������Ũ��c��SO32-����
��3������Ϣ��֪�����ı�I2��Һ�����Ϊ$\frac{15.98mL+16.02mL}{2}$=16.0mL�����SO2+I2+2H2O�TH2SO4+2HI�����������������Դ������
��� �⣺��1�������������ΪxmL���ɵ����غ��֪��$\frac{1.90}{190g/mol}$��2����6-4��=$\frac{x��1{0}^{-3}L}{22.4L/mol}$��2����2-0�������x=224��
�ʴ�Ϊ��224��
��2������ҺpH=4.5����Һ�����ԣ���ͼ��֪����������HSO3-����Na2S2O5�ܽ���ˮ�Ļ�ѧ����ʽΪNa2S2O5+H2O=2NaHSO3������ҺpHС��1����Һ��H2SO3��Ũ�ȱ�С����ԭ�������������ȶ����ֽ����ɶ������������ᱻ��������Ksp[BaSO4]=c��Ba2+��•c��SO42-������֪��Ҫc��Ba2+��=$\frac{1��1{0}^{-10}}{1��1{0}^{-5}}$=10-5mol•L-1������Һ��SO32-�����Ũ��c��SO32-��=$\frac{5��1{0}^{-7}}{1{0}^{-5}}$=0.05mol•L-1��
�ʴ�Ϊ��Na2S2O5+H2O=2NaHSO3��������ȶ����ֽ����ɶ������������ᱻ������0.05��
��3������Ϣ��֪�����ı�I2��Һ�����Ϊ$\frac{15.98mL+16.02mL}{2}$=16.0mL������I2�����ʵ���Ϊ16.0��10-3L��0.0225mol•L-1=3.6��10-4mol�����ݷ�ӦSO2+I2+2H2O=H2SO4+2HI����֪������������ʵ���Ϊ3.6��10-4mol��SO2������Ϊ64g/mol��3.6��10-4mol=23.04mg���������Ѿ���Ʒ�п��������IJ�����Ϊ$\frac{23.04mg}{0.1L}$=230.4mg•L-1��
�����Ѿ���Ʒ�п��������IJ�����Ϊ230.4mg•L-1��
���� ���⿼���ﺬ�����㡢������ԭ��Ӧ���㼰ͼ�������Ϊ��Ƶ���㣬���շ����ķ�Ӧ�����ʵ����Ĺ�ϵ��Ϊ���Ĺؼ������ط�������������Ŀ��飬��Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ��װ�� | ���� |
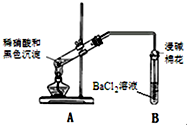 | 1��A�Թ��к�ɫ�������ܽ� 2��A�Թ����Ϸ����ֺ���ɫ���� 3��B�Թ��г��ְ�ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
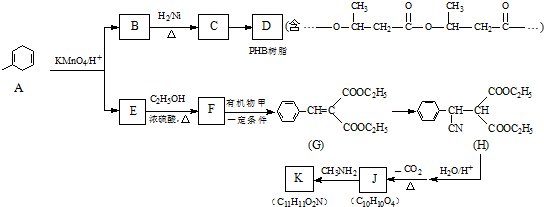
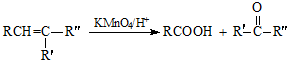
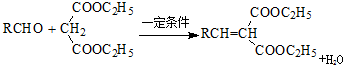
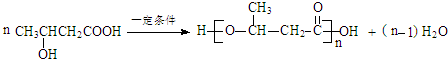 ����
���� ��
��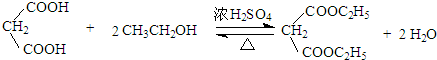 ��
��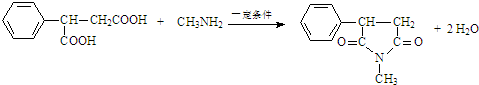 ��
��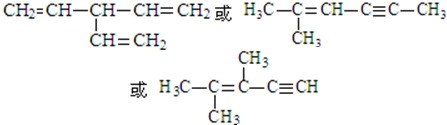 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 100g•mol-1 | B�� | 108g•mol-1 | C�� | 55g•mol-1 | D�� | 96g•mol-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
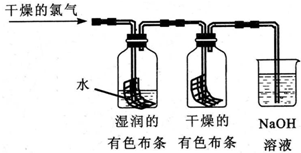
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 1807�껯ѧ�Ҵ�ά�õ���������������Ƶ��ƣ�4NaOH$\frac{\underline{\;ͨ��\;}}{\;}$4Na+O2��+2H2O��������•����������������������������Ҳ�Ƶ��ƣ���Ӧԭ��Ϊ��3Fe+4NaOH$\frac{\underline{\;1100��\;}}{\;}$Fe3O4+2H2��+4Na���������й�˵����ȷ���ǣ�������
1807�껯ѧ�Ҵ�ά�õ���������������Ƶ��ƣ�4NaOH$\frac{\underline{\;ͨ��\;}}{\;}$4Na+O2��+2H2O��������•����������������������������Ҳ�Ƶ��ƣ���Ӧԭ��Ϊ��3Fe+4NaOH$\frac{\underline{\;1100��\;}}{\;}$Fe3O4+2H2��+4Na���������й�˵����ȷ���ǣ�������| A�� | ������������������ƣ����������ĵ缫��ӦΪ��2OH--2e-�TH2��+O2�� | |
| B�� | ��•�����˷�����֤�����Ļ�ԭ�Ա���ǿ | |
| C�� | ���ô�ά�����•�����˷��Ƶõ������ƣ�������Ӧ����ת�Ƶĵ�������ͬ | |
| D�� | ��������Ȼ������Ƶĵ����У�����ͼ����ʯīΪ��������Ϊ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
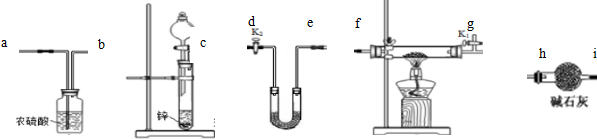
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
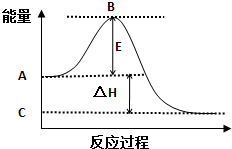 2SO2��g��+O2��g���T2SO3��g����Ӧ���̵������仯��ͼ��ʾ����֪1mol SO2��g������Ϊ1mol SO3��g���ġ�H=-99kJ/mol��
2SO2��g��+O2��g���T2SO3��g����Ӧ���̵������仯��ͼ��ʾ����֪1mol SO2��g������Ϊ1mol SO3��g���ġ�H=-99kJ/mol���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ͬһ�ܲ��px��py��pz�������������ͬ | |
| B�� | 3d3��ʾ3d�ܼ���3����� | |
| C�� | p�������������һ������s����������� | |
| D�� | ���������������˶��ĵ���������� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com