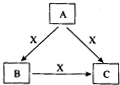
A��B��C��X����ѧ��ѧ�������ʣ�ת����ϵ��ͼ��ʾ����������²�ͬ����ش����⣺
��1����A��B��C�о���ͬһ�ֳ�������Ԫ�أ���Ԫ����C������������ʽ���ڣ���A��C��ˮ��Һ��Ͽɵ�B�İ�ɫ������
��A��B��C�������Ľ���Ԫ��Ϊ��д���ƣ�
��
��
��A��C��ˮ��Һ�з�Ӧ�����ӷ���ʽ��
Al3++3AlO2-+6H2O�T4 Al��OH��3��
Al3++3AlO2-+6H2O�T4 Al��OH��3��
��
�ڵ������ĸý���Ԫ�ص��ʷֱ����������ᡢ����������Һ��Ӧ���������������֮�ȣ�ͬ��ͬѹ����
1��1
1��1
���������ᡢ�����������ʵ���֮����
3��1
3��1
��
��2����AΪ���з�����ȼ�����ʵ�ϡ��Һ��X�Ǿ���Ư���Ե���ɫ���壬Aת��ΪB��ͬʱ���õ���һ�ִ�����ɵĽ��壬��B��
Na2SO3
Na2SO3
��Aת��ΪC�����ӷ���ʽ��
SiO32-+2SO2+2H2O�TH2SiO3�����壩+2HSO3-
SiO32-+2SO2+2H2O�TH2SiO3�����壩+2HSO3-
��X��ʹ��ˮ��ɫ���䷴Ӧ�����ӷ���ʽ��
Br2+SO2+2H2O�T4H++2Br-+SO42-
Br2+SO2+2H2O�T4H++2Br-+SO42-
��3����A��B��C����ɫ��Ӧ���ʻ�ɫ��ˮ��Һ��Ϊ���ԣ�x��һ���������壬��A��һ�ֳ���ǿ�
�ٹ�ҵ������C�Ļ�ѧ��Ӧ����ʽ��
NaCl+NH3+CO2+H2O�TNaHCO3��+NH4Cl
NaCl+NH3+CO2+H2O�TNaHCO3��+NH4Cl
��
�ڹ�ҵ����C�õ�B�Ļ�ѧ��Ӧ����ʽΪ
��
����Ȼ������B��C��H
2O��һ�������ᾧ���ɵĹ���W��ȡһ����W����ˮ���100mL��Һ����������н��������ӵ�Ũ��Ϊ0.5mol/L����ȡ��ͬ������W���������أ�ʣ����������Ϊ
2.65g
2.65g
��

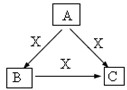 A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��������������ֲ�ͬ����ش�
A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��������������ֲ�ͬ����ش� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�� OH-+HCO3-���ʴ�Ϊ��CO32-+H2O
OH-+HCO3-���ʴ�Ϊ��CO32-+H2O OH-+HCO3-��
OH-+HCO3-��


 A��B��C��X����ѧ��ѧ�������ʣ�ת����ϵ��ͼ��ʾ����������²�ͬ����ش����⣺
A��B��C��X����ѧ��ѧ�������ʣ�ת����ϵ��ͼ��ʾ����������²�ͬ����ش����⣺ A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��ʾ����������²�ͬ����ش�
A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��ʾ����������²�ͬ����ش� A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��ʾ��������������ֲ�ͬ����ش�
A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��ʾ��������������ֲ�ͬ����ش�

 A��B��C��X����ѧ��ѧ�г��������ʣ�����֮���ת����ϵ��ͼ��ʾ�����ֲ�������ȥ������ش��������⣺
A��B��C��X����ѧ��ѧ�г��������ʣ�����֮���ת����ϵ��ͼ��ʾ�����ֲ�������ȥ������ش��������⣺ A��B��C��X����ѧ��ѧ�г��������ʣ�����֮���ת����ϵ����ͼ��ʾ�����ֲ�������ȥ������ش��������⣺
A��B��C��X����ѧ��ѧ�г��������ʣ�����֮���ת����ϵ����ͼ��ʾ�����ֲ�������ȥ������ش��������⣺