
| A��Y2���Ļ�ԭ�Խ�Z��ǿ |
| B��Y���⻯����X2Y������ѧ����������ͬ |
| C��YO2��Z2������ΪƯ��������Ư�����õ�ԭ����ͬ |
| D����ʯīΪ�缫���XZ��ˮ��Һ�����������������̪�ɹ۲쵽��Һ�Ժ�ɫ |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������ƾ��ǵ����˶�����̬ |
| B����ɫ��Ӧ�������Ƿ������ |
| C��ͭԭ�Ӵ��ڻ�̬ʱ�ĺ�������Ų�ʽΪ3d104s1 |
| D����������˶�״̬������ţ���˶�������������Ҳ������ͳ�Ʒ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��NH4Br�ĵ���ʽ�� |
B��S2-�Ľṹʾ��ͼ�� |
| C������ķ���ʽ��CH3COOH |
D��ԭ�Ӻ�����18�����ӵ���ԭ�ӣ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������Ʒ¶�����һ��ʱ�䣬������ǿ����������ˮ�����ɷֱ�������ԭ�� |
| B��CH4��NH3��PH3�����ȶ�������ǿ |
| C����Ϊ���ԣ�HC1>HF�����Էǽ����ԣ�Cl>F |
| D����ԭ���ڷ�Ӧ��ʧȥ�ĵ��ӱ���ԭ���٣����ƵĽ����Ա����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
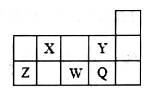
| A��Ԫ�صķǽ�����Y��Q������ͬŨ���⻯��ˮ��Һ�����ԣ�HY��HQ |
| B���γɼ����ӵİ뾶��С�����˳����Y��Q��W |
| C��Z����������������ˮ��Ҳ������ǿ����Һ |
| D��X����̬�⻯��Ũ��Һ�����ڼ���ܵ�������й© |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���
| AԪ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�ء� |
| BԪ��ԭ�ӵĺ���p��������s��������1�� |
| Cԭ�ӵĵ�һ�����ĵ����ֱܷ��ǣ�I1=738kJ/mol I2=1451J/mol I3=7733kJ/mol I4=10540kJ/mol |
| Dԭ�Ӻ�������p���ȫ��������� |
| EԪ�ص������������������IJ�Ϊ4�� |
| F��ǰ�������е縺����С��Ԫ�ء� |
| G�����ڱ��ĵ����С� |
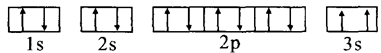
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A�������ж�����Ԫ�����ʵ������ƶ�Ԫ�آ�����������Ӧ��ˮ���������ǿ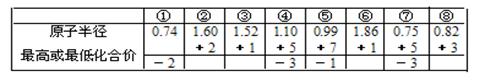 |
B��ͼ�ױ�ʾZn��Cuԭ��ط�Ӧ�����еĵ���ǿ�ȵı仯��Tʱ���ܼ�����H2O2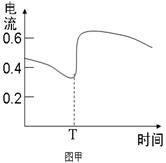  |
| C��ͼ�ұ�ʾijһ���ȷ�Ӧ����ʹ�ô���E1��E2����H���ᷢ���ı� |
| D����ʾH2ȼ���ȵ��Ȼ�ѧ����ʽΪ��2H2��g��+O2��g��=2H2O��l������H= -571.6kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���
| Ԫ�� | �����Ϣ |
| X | �ؿ��к������Ľ�������������������ͻ���� |
| Y | �����ڳ��³�ѹ��Ϊ����ɫ���� |
| Z | ���³�ѹ�£������ǵ���ɫ���壬���ڻ�ɽ�ڸ������� |
| U | �����µ�����Ũ����ۻ�����һ�ֺ��ص�������Ϊ56��������Ϊ30 |
| V | ��������Ԫ�أ�ԭ�ӵ��������������ڲ��������2/5 |
 U3++3H2O��ƽ�ⳣ��K= ��
U3++3H2O��ƽ�ⳣ��K= ���鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com