
��֪H2A��ˮ�д�������ƽ�⣺H2A H����HA����HA��
H����HA����HA�� H����A2�����ش��������⣺
H����A2�����ش��������⣺
��1����֪�����£�H2A�ĸ���(CaA)������Һ�д���ƽ�⣺
CaA(s) Ca2��(aq)��A2��(aq)����H �� 0��
Ca2��(aq)��A2��(aq)����H �� 0��
���¶�����ʱ��Ksp________(���������С�����䡱��ͬ)��
�ڵμ�����Ũ���ᣬc (Ca2��)________��ԭ����____ ______________(�����ֺ����ӷ���ʽ˵��)��
��2������CaA����Һ�м���CuSO4��Һ������һ�ֺ�ɫ�������ʣ�д���ù����з�Ӧ�����ӷ���ʽ______________________����ijCuSO4��Һ��c (Cu2��)��0.02 mol/L�����Ҫ����Cu(OH)2������Ӧ������ҺpH��ʹ֮����________(��֪Ksp[Cu(OH)2]��2.0��10��20)��
��3�������£���ij������Һ�е����̪����Һ�ʺ�ɫ�������Һ��________�ԡ��ڷ�������Һ����̪�ʺ�ɫ��ԭ��ʱ����ͬѧ��Ϊ��������Һʱ���õĴ�����Ʒ�л���NaOH���£���ͬѧ��Ϊ����Һ�е������CO32-ˮ�����£��������һ����ʵ�鷽����������λͬѧ��˵����������(������Ҫ����������ͽ���)_________________ ___________��
��1������������ �����ᷢ����Ӧ��A2����H�� HA����A2��Ũ�ȼ�С��CaA���ܽ�ƽ�������ƶ���n(Ca2��)����������Һ����仯��������c(Ca2��)����
HA����A2��Ũ�ȼ�С��CaA���ܽ�ƽ�������ƶ���n(Ca2��)����������Һ����仯��������c(Ca2��)����
��2��CaA(s)��Cu2��(aq)=Ca2��(aq)��CuA(s) ��2�֣� 5��2�֣�
��3���� �� ����Ba2����ȥ̼������ӵĸ��ţ������Һ���Ժ�ɫ����֤����ͬѧ�Ĺ۵���ȷ���������̼���ˮ���Ե�ʣ�����ͬѧ�Ĺ۵���ȷ��������������Ҳ���֣�
���������������1����֪�����£�H2A�ĸ���(CaA)������Һ�д���ƽ�⣺CaA(s)? ?Ca2��(aq)��A2��(aq)����H �� 0���ٸ÷�ӦΪ���ȷ�Ӧ���¶����ߣ�ƽ�������ƶ���Ksp���ڸ��ݻ�ѧƽ���ƶ�ԭ�����μ�����Ũ���ᣬ������Ӧ��A2����H��
?Ca2��(aq)��A2��(aq)����H �� 0���ٸ÷�ӦΪ���ȷ�Ӧ���¶����ߣ�ƽ�������ƶ���Ksp���ڸ��ݻ�ѧƽ���ƶ�ԭ�����μ�����Ũ���ᣬ������Ӧ��A2����H�� HA����A2��Ũ�ȼ�С��CaA���ܽ�ƽ�������ƶ���n(Ca2��)����������Һ����仯��������c(Ca2��)����2��CaA����Һ�м���CuSO4��Һ���������ֽⷴӦ������һ�ֺ�ɫ��������CuA�����ӷ���ʽΪCaA��s��+Cu2+��aq��?Ca2+��aq��+CuA��s�������Ҫ����Cu(OH)2����������c��Cu2+����c2��OH������Ksp[Cu(OH)2] ��2.0��10��20��c (Cu2��)��0.02 mol/L����c��OH������10��9��Ӧ������ҺpH��ʹ֮����5����3�������£���ij������Һ�е����̪����Һ�ʺ�ɫ�������Һ�ʼ��ԣ�ʵ�鷽��Ϊ������Ba2����ȥ̼������ӵĸ��ţ������Һ���Ժ�ɫ����֤����ͬѧ�Ĺ۵���ȷ���������̼���ˮ���Ե�ʣ�����ͬѧ�Ĺ۵���ȷ��
HA����A2��Ũ�ȼ�С��CaA���ܽ�ƽ�������ƶ���n(Ca2��)����������Һ����仯��������c(Ca2��)����2��CaA����Һ�м���CuSO4��Һ���������ֽⷴӦ������һ�ֺ�ɫ��������CuA�����ӷ���ʽΪCaA��s��+Cu2+��aq��?Ca2+��aq��+CuA��s�������Ҫ����Cu(OH)2����������c��Cu2+����c2��OH������Ksp[Cu(OH)2] ��2.0��10��20��c (Cu2��)��0.02 mol/L����c��OH������10��9��Ӧ������ҺpH��ʹ֮����5����3�������£���ij������Һ�е����̪����Һ�ʺ�ɫ�������Һ�ʼ��ԣ�ʵ�鷽��Ϊ������Ba2����ȥ̼������ӵĸ��ţ������Һ���Ժ�ɫ����֤����ͬѧ�Ĺ۵���ȷ���������̼���ˮ���Ե�ʣ�����ͬѧ�Ĺ۵���ȷ��
���㣺����������Һ�������ܽ�ƽ�⡣


 �γ̴����Ծ�����100��ϵ�д�
�γ̴����Ծ�����100��ϵ�д� �¾�����ĩ���100��ϵ�д�
�¾�����ĩ���100��ϵ�д� ȫ�ܴ���100��ϵ�д�
ȫ�ܴ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
25 ��ʱ,����ƽ�ⳣ��:
| ����Ļ�ѧʽ | CH3COOH | HClO | H2CO3 |
| ����ƽ�ⳣ��(25 ��) | 1.8��10-5 | 3.0�� | K1=4.3��10-7 K2=5.6��10-11 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ʵ�����������õ���Ⱥ͵���ƽ�ⳣ����ʾ����1�dz����¼�������ĵ���ƽ�ⳣ��(Ka)������ĵ���ƽ�ⳣ��(Kb)����2�dz����¼�����()������ܶȻ�����(Ksp)��
��1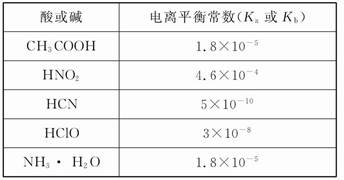
��2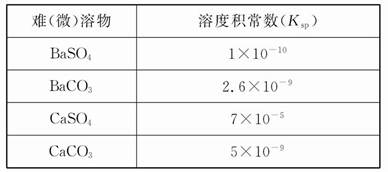
��ش��������⣺
(1)CH3COONH4��ˮ��Һ�� (ѡ����ԡ��������ԡ����ԡ�)�������� ����Һ�и�����Ũ�ȴ�С��ϵ�� ��
(2)���ʵ���֮��Ϊ1��1��NaCN��HCN�Ļ����Һ����pH>7������Һ������Ũ�ȴӴ�С������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijͬѧ�ù�ҵ����ͭ�����������������ʣ��Ʊ�������CuSO4��5H2O��������������
�����ֲ����������ԣ���
I��ȡ��ҵ����ͭ���壬��ϡ�����ܽ⣬���ˡ�
II������Һ�еμ�H2O2��Һ���Լ��ȡ�
III����II����Һ�м���CuO��ĩ��pH��4��
IV��������У����ˣ���Һ��ϡ�����ữ��pH��1��
V������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����þ��塣
��֪�����������������������pH��Ksp��25�棩���±���
| ���� | Fe(OH)3 | Fe (OH)2 | Cu(OH)2 |
| ��ʼ����ʱpH | 2.7 | 7.6 | 4.7 |
| ��ȫ����ʱpH | 3.7 | 9.6 | 6.7 |
| Ksp | 4.0��10�C38 | 8.0��10�C16 | 2.2��10�C20 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������������ճ������м�Ϊ�������ᣬ��һ��������CH3COOH��Һ�д��ڵ���ƽ�⣺CH3COOH CH3COO��+H+ ��H��0
CH3COO��+H+ ��H��0
��1�������£�pH��5������Һ�У�c(CH3COO��)��______mol/L(��ȷֵ��Ҫ����ʽ���ػ���)��
��2�����з����п���ʹ0.10 mol��L-1 CH3COOH�ĵ���̶��������
a����������0.10 mol��L��1ϡ���� b������CH3COOH��Һ c����ˮϡ����0.010 mol��L��1
d���������������� e�����������Ȼ��ƹ��� f����������0.10 mol��L��1 NaOH��Һ
��3������������пͶ��������pH������3�Ĵ����������Һ�У�������ַ�Ӧ����ֻ��һ����Һ����п��ʣ�࣬�����������������V(����)_________V(����)������ڡ�����С�ڡ����ڡ���
��4����NaOH��Һ�ֱ�ζ�20.00mL0.1mol/L�����20.00mL0.1mol/L������Һ���õ���ͼ��ʾ�����ζ����ߣ���NaOH��Һ�ζ�������Һ�������� ���ͼ1����ͼ2����
��5�������£���0.1 mol/L�����0.1 mol/L��������Һ��ϣ�������ҺΪ���ԣ�������Һ�и����ӵ�Ũ�Ȱ��ɴ�С����Ϊ_______________________________��
��6����ͼ��ʾ��Һ��c(H��)��c(OH��)�Ĺ�ϵ
��M�����ڣ���Ӱ���֣������c(H��)______c(OH��)������ڡ�����С�ڡ����ڡ���
����T2�¶��£���pH��9 NaOH��Һ��pH��4 HCl��Һ��ϣ������û����Һ��pH��7����NaOH��Һ��HCl��Һ�������Ϊ ������Ϻ���Һ����ı仯���Բ��ƣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����£���Ũ�Ⱦ�Ϊ1 mol��L��1������4����Һ��
��H2SO4��Һ ��NaHCO3��Һ ��NH4Cl��Һ ��NaOH��Һ
��1����4����ҺpH�ɴ�С��˳���� ��������ˮ�����H��Ũ����С���� ����������ţ�
��2�����и�����Ũ���ɴ�С��˳���� ��NaHCO3��ˮ��ƽ�ⳣ��Kh�� mol��L��1������֪̼��ĵ��볣��K1��4��10��7��K2��5.6��10��11��
��3�������ͨ��������������ʱ ��ֵ �����������С�����䡱����
��ֵ �����������С�����䡱����
��4�������ۺܻ͢�Ϻ���Һǡ�ó����ԣ�����ǰ�۵���� �ܵ����������ڡ�����С�ڡ����ڡ�֮һ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����ܽ�ƽ��Ҳ��ƽ�ⳣ������Ϊ�ܶȻ�����������ΪKsp������ij�����ܽ�ƽ�⣺
MmAn��s�� mMn+��aq�� + nAm����aq����Ksp��[c��Mn+��]m��[c��Am����]n��
mMn+��aq�� + nAm����aq����Ksp��[c��Mn+��]m��[c��Am����]n��
��1������ʱ��Fe��OH��3��Ksp��1��10��38��Ҫʹ��Һ�е�Fe3+������ȫ����������Һ�е�c��Fe3+����10��5 mol��L��1��������Һ��pH��СΪ ��
��2��ij�¶�ʱ����100gˮ���ܽ�0.74g Ca��OH��2�ﵽ���ͣ���ʱ��Һ�����ԼΪ100 mL������¶���Ca��OH��2���ܶȻ�����Ksp�� ����д��������̣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1��������pH="5" HCl��Һ��pH=5��AlCl3��Һ����ˮ���������c (H+) ֮�ȵ��� ��
��2��д����ĭ��������ʱ������Ӧ�����ӷ���ʽ ��
��3�������½�0.01molCH3COONa��0.02mol��������ˮ�����0.5L�����Һ����Һ�й��� ������������Ũ�ȴӴ�С��˳��Ϊ ��
��4�������£���100 mL 0.01 mol��L��1HA��Һ��μ���0.02 mol��L��1MOH��Һ��ͼ����ʾ���߱�ʾ�����Һ��pH�仯�����������Һ���ʱ������仯����
�ش��������⣺
����ͼ����Ϣ��֪HAΪ_______�ᣨ�ǿ���������� �� K���Ӧ����Һ��c(M+)��c(MOH)= mol��L��1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1��25��ʱ��ijNaCl��Һ��c(Cl�C)��1��10��4 mol��L�C1�������Һ��c(Na��)��c(OH��)��
��2��25��ʱ����0.1 mol��L�C1NaOH��Һ��0.06 mol��L�C1��H2SO4��Һ��������(���Ի�Ϻ�����ı仯)����������Һ��pH�� ��25��ʱ��pHֵΪ8��NaOH��Һ��pHֵΪ10��NaOH��Һ�������Ϻ���Һ��������Ũ����ӽ� ��
��3��25��ʱ������������Һ�У���pH=0������ ��0.1 mol��L�C1������ ��0.01 mol��L�C1��NaOH��Һ ��pH=11��NaOH��Һ����ˮ��������������Ũ��֮�Ȣ٩U�کU�۩U���ǣ� (����ĸ)
| A��1�U10�U100�U1000 | B��0�U1�U12�U11 |
| C��14�U13�U12�U11 | D��14�U13�U2�U3 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com