
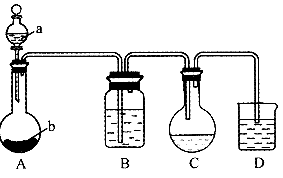
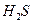 ��NaOH��Ӧ�ܿ졣
��NaOH��Ӧ�ܿ졣 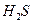 ��NaOH��Ӧ�ܿ졣
��NaOH��Ӧ�ܿ졣 

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ü�ʽ�ζ���ȷȡ��25��00mL 1 mol��L���������Һ |
| B�������������������������Һ��ϼ��ȣ���ȴ��� ��������Һ������������Ԫ�� |
| C�����������ƹ�����ϡ�����ϣ��������ǰ���¶ȱ仯�� ֤���кͷ�Ӧ�Ƿ��ȷ�Ӧ |
| D������ͼװ�ø��ﰱ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ҩƷ���ͬһ�㲻�ɴ��Ũ�����Ũ��ˮ |
| B���������Ʊ�������ˮ�Ҵ��� |
| C���������ױ����ڶ���̼�� |
| D����������Һ�����ڲ��������Լ�ƿ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A��������������ж������ռ���������������������ɢ�������У���Ⱦ������ |
| B���ڴ��ε��ᴿʵ���У��ܽ⡢���ˡ��������õ��˲����� |
| C���ᡢ������У�������ˮ������ϴ��������գ�ۺ����� |
| D������FeCl3��Һʱ����FeCl3�������ڽ�Ũ�����У�Ȼ������ˮϡ�͵������Ũ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���Ʊ���������ʱ�����Ҵ����������μ��뵽Ũ������ |
| B��������պȡ��Һ�ε����ڱ������ϵ�pH��ֽ�� |
| C������5%ʳ����Һʱ����������ʳ�η����ձ��мӼ�����ˮ�����ܽ� |
| D�������Ȼ�������Һʱ����һ�����Ȼ����ܽ��ڽ�Ũ�������У��ټ��������ۺ���ˮϡ�͵�����Ũ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ۢݢ� | B���ڢ� | C���٢ڢ� | D���٢ڢۢܢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ���ڲ����ܿ�����һ��ʪ��ĺ�ɫʯ����ֽ����ش��������⣺
���ڲ����ܿ�����һ��ʪ��ĺ�ɫʯ����ֽ����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
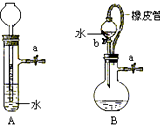
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������ɫƿ�� | B������CCl4�� |
| C������ˮ�� | D������ú���� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com