
��A��B���������������Ϣ���£�
| A | ����ȫȼ�յIJ�����n(CO2)��n(H2O)��2��1 ��28��Mr(A)��60 �۲���ʹ������Ȼ�̼��Һ��ɫ ��һ�ȴ���ֻ��һ�ֽṹ |
| B | �ٱ���������ͨ������³���̬ ����ͬ���칹�� �۶������������ |
�ش��������⣺
(1)��A�����ʽ��________��
(2)��A�Ľṹ��ʽ��_____________________________________________________��
(3)��B�����ֶ������Ľṹ��ʽΪ_______________________________________��
(4)��CΪ��B��ͬϵ�������Ϊ��̬��ֻ��һ��һ��������C��һ�����Ľṹ��ʽΪ________(��һ�ּ���)��
������(1)��A��ȫȼ�յIJ�����n(CO2)��n(H2O)��2��1������A�ķ���ʽΪ(CH)n��
(2)��28��Mr(A)��60��������A�ķ���ʽΪC3H3��C4H4���������������Ϣ��֪��A�Ľṹ��ʽ�� ��
��
(3)��BΪ����������ͨ������³���̬������BΪCH4��C2H6��C3H8��C4H10����ͬ���칹�壬����ΪCH4��C2H6��C3H8��������BΪC4H10���������ֶ������Ľṹ��ʽ�ֱ�Ϊ
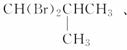
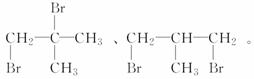
(4)��B��ͬϵ���г�����Ϊ��̬����CH4��C2H6��C3H8��C3H8������һ����CH4��C2H6��ֻ��һ��һ�����ֱ�ΪCH3Br��C2H5Br��
�𰸡�(1)CH��(2) ��(3)
��(3) 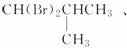
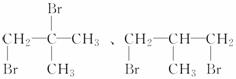
(4)CH3Br(��C2H5Br)


 ������ϰ�ο����뵥Ԫ���ϵ�д�
������ϰ�ο����뵥Ԫ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������ʵ�鷽�����Լ����ܴﵽʵ��Ŀ�ĵ���(����)
A������SO2��CO2���壺�ó���ʯ��ˮ
B��֤����Һ�к���NH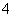 ����ŨNaOH��Һ����ɫʯ����ֽ
����ŨNaOH��Һ����ɫʯ����ֽ
C������Br����I����˫��ˮ�͵�����Һ
D������NO2����������������ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ư������Һ�д�������ƽ�⣺ClO����H2O HClO��OH�������д�ʩ�������Ư��Ч�ʵ���
HClO��OH�������д�ʩ�������Ư��Ч�ʵ���
A����H2O�������������� B��ͨ��CO2
C��ͨ��SO2 D��������NaOH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)����ʱ��������Һ����.pH��4��CH3COOH��Һ����.pH��4��HCl��Һ����.pH��10��NaOH��Һ����.pH��10��CH3COONa��Һ��
�٢�͢�����Һ��ˮ�����c(H��)Ũ��֮��_____________________________________��
�������й�������ȷ����________��
A�������Һ�ֱ���10 g���۷�Ӧ������H2���������
B������͢�������Ϻ���ҺpHС��7
C��������Һ��10 mL�ֱ��ˮϡ����100 mL����Һ��pH������ ��
��
(2)CH3COOH��Һ��Ka��1.6��10��5����1.0 mol��L��1��CH3COONa��Һ ��c(OH��)��________________________________________________________________________��
��c(OH��)��________________________________________________________________________��
(3)��CO2ͨ��NaOH��Һ�У��ش��������⡣
�ٵ�CO2��NaOH���ʵ���֮��Ϊ1��2ʱ����Һ������Ũ�ȵĴ�С˳��Ϊ________________________________________________________________________��
�ڵ�c(Na��)��c(CO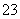 )��c(HCO
)��c(HCO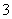 )��c(H2CO3)ʱ����Ӧ����Һ�е�����Ϊ________��
)��c(H2CO3)ʱ����Ӧ����Һ�е�����Ϊ________��
(4)��NaOH��Һ�ζ�������Һ�Ĺ����У���ƿ�е���Һ������Ũ�ȹ�ϵ������ʽ�ӣ��ڵζ���������Щʽ�ӳ��ֵ��Ⱥ�˳��Ϊ________(��ѡ����ȷ��˳��)��
��c(Na��)��c(CH3COO��)��c(OH��)��c(H��)
��c(CH3COO��)��c(Na��)��c(H��)��c(OH��)
��c(CH3COO��)��c(H��)��c(Na��)��c(OH��)
��c(Na��)��c(CH3COO��)��c(OH��)��c(H��)
��c(CH3COO��)��c(H��)��c(Na��)��c(OH��)
��c(Na��)��c(CH3COO��)��c(OH��)��c(H��)
��c(Na��)��c(OH��)��c(CH3COO��)��c(H��)
A���ߢܢ٢ޢڢۢݡ����� B���ݢڢۢ٢ޢܢ�
C���ۢݢڢ٢ޢܢ� D���ݢۢڢޢ٢ܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ����
A���л�����������Ҫԭ�����л�����ӽṹʮ�ָ���
B����������е�̼ԭ������ԭ����ͨ���Ǽ��Լ���ϵ�
C��ͬ���칹����Ĺ㷺����������л���������Ψһԭ��
D�������Ľṹ�ص���̼ԭ��ͨ���������ӳ���״��ʣ��ۼ�������ԭ�ӽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����л��������зе���ߵ���
A�����顡�������������� B����ϩ
C���Ҵ� D������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й����ʽṹ��������ȷ����
A���ױ������е�����ԭ�ӿ��ܹ�ƽ��
B��CH2===CH��C6H5�����е�����ԭ�ӿ��ܹ�ƽ��
C����������е�����ԭ�ӿ��ܹ�ƽ��
D�����ȼ������Ϊ��������ṹ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и������ʲ�����һ���Լ�ͨ����ѧ��Ӧ���ֵ���(����)
A��MnO2��������CuO��������FeO
B��(NH4)2SO4 K2SO4 NH4Cl
C��AgNO3 KNO3 Na2SO3
D��Na2CO3 NaHCO3 K2CO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������У��Ⱥ��м��Լ����ֺ��зǼ��Լ����ǣ� ��
A������C6H6�� B��CO2 C��Na2O2 D��NH4Cl
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com