
(1)����ʱ��������Һ����.pH��4��CH3COOH��Һ����.pH��4��HCl��Һ����.pH��10��NaOH��Һ����.pH��10��CH3COONa��Һ��
�٢�͢�����Һ��ˮ�����c(H��)Ũ��֮��_____________________________________��
�������й�������ȷ����________��
A�������Һ�ֱ���10 g���۷�Ӧ������H2���������
B������͢�������Ϻ���ҺpHС��7
C��������Һ��10 mL�ֱ��ˮϡ����100 mL����Һ��pH������ ��
��
(2)CH3COOH��Һ��Ka��1.6��10��5����1.0 mol��L��1��CH3COONa��Һ ��c(OH��)��________________________________________________________________________��
��c(OH��)��________________________________________________________________________��
(3)��CO2ͨ��NaOH��Һ�У��ش��������⡣
�ٵ�CO2��NaOH���ʵ���֮��Ϊ1��2ʱ����Һ������Ũ�ȵĴ�С˳��Ϊ________________________________________________________________________��
�ڵ�c(Na��)��c(CO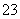 )��c(HCO
)��c(HCO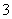 )��c(H2CO3)ʱ����Ӧ����Һ�е�����Ϊ________��
)��c(H2CO3)ʱ����Ӧ����Һ�е�����Ϊ________��
(4)��NaOH��Һ�ζ�������Һ�Ĺ����У���ƿ�е���Һ������Ũ�ȹ�ϵ������ʽ�ӣ��ڵζ���������Щʽ�ӳ��ֵ��Ⱥ�˳��Ϊ________(��ѡ����ȷ��˳��)��
��c(Na��)��c(CH3COO��)��c(OH��)��c(H��)
��c(CH3COO��)��c(Na��)��c(H��)��c(OH��)
��c(CH3COO��)��c(H��)��c(Na��)��c(OH��)
��c(Na��)��c(CH3COO��)��c(OH��)��c(H��)
��c(CH3COO��)��c(H��)��c(Na��)��c(OH��)
��c(Na��)��c(CH3COO��)��c(OH��)��c(H��)
��c(Na��)��c(OH��)��c(CH3COO��)��c(H��)
A���ߢܢ٢ޢڢۢݡ����� B���ݢڢۢ٢ޢܢ�
C���ۢݢڢ٢ޢܢ� D���ݢۢڢޢ٢ܢ�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪NaHSO4��ˮ�еĵ��뷽��ʽΪ��NaHSO4===Na����H����SO ��ij�¶��£���pH��6������ˮ�м���NaHSO4���壬�����¶Ȳ��䣬�����Һ��pHΪ2�����ڸ���Һ�����������в���ȷ����
��ij�¶��£���pH��6������ˮ�м���NaHSO4���壬�����¶Ȳ��䣬�����Һ��pHΪ2�����ڸ���Һ�����������в���ȷ����
A�����¶��¼�������pH��12��NaOH��Һ��ʹ��Ӧ�����Һǡ��������
B��ˮ���������c(H��)��1��10��10 mol��L��1
C��c(H��)��c(OH��)��c(SO )
)
D�����¶ȸ���25 ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����CO2��SO2����ѷ����ǡ������� �� ��
A.��Ʒ����Һ B�ó����ʯ��ˮ C������ͭ��Һ D.��ʪ��ĺ�ɫʯ����ֽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������̼���ƻ�̼�������ܷ���ˮ�����ʵ�ص���
A��ʵ����ʢ��̼������Һ���Լ�ƿ������������ �����ò�����
��������
B����ĭ�������̼��������Һ����������Һ��ʹ��ʱֻ�轫���ϾͿɲ�������������̼����ĭ
C�������г���̼������Һϴ�Ӳ;��ϵ�����
D������̼�����������ȡ����������̼
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����a mol��L��1NaX��b mol��L��1NaY��������Һ������˵����ȷ����(������Һ��ϣ�����Ի��ʱ������仯)
A����a��b��pH(NaX)��pH(NaY)��������HX��HY
B����a��b��c(X��)��c(Y��)��c(HY)��������HX��HY
C����a��b��c(X��)��c(Y��)��������HX��HY
D����a��0.1 mol��L��1������Һ�������ϣ���c(X��)��c(HX)��0.1 mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�л���A�Ľṹ����ͼ��ʾ��A���б�����ͬ���칹���в������������
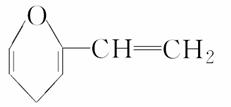
A���� B��ȩ
C���� D����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��A��B���������������Ϣ���£�
| A | ����ȫȼ�յIJ�����n(CO2)��n(H2O)��2��1 ��28��Mr(A)��60 �۲���ʹ������Ȼ�̼��Һ��ɫ ��һ�ȴ���ֻ��һ�ֽṹ |
| B | �ٱ���������ͨ������³���̬ ����ͬ���칹�� �۶������������ |
�ش��������⣺
(1)��A�����ʽ��________��
(2)��A�Ľṹ��ʽ��_____________________________________________________��
(3)��B�����ֶ������Ľṹ��ʽΪ_______________________________________��
(4)��CΪ��B��ͬϵ�������Ϊ��̬��ֻ��һ��һ��������C��һ�����Ľṹ��ʽΪ________(��һ�ּ���)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʽΪC3H4Cl2�Һ���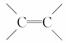 �ṹ���л����ͬ���칹�干��(�����������칹)
�ṹ���л����ͬ���칹�干��(�����������칹)
A��3�� B��4��
C��5�� D��6��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���з�Ӧ�У�ˮ�Ȳ�����������Ҳ������ԭ����������ԭ��Ӧ�ǣ� ��
A��Na��H2O B��Mg ��H2O C��CO2��H2O D��Na2O2 ��H2O
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com