
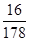 ��32g��������2mol���飬�����ͷŵļ���������ȫȼ������CO2��Һ̬ˮ���ų�������Ϊ889.6kJ/mol��2mol��1779.2kJ��
��32g��������2mol���飬�����ͷŵļ���������ȫȼ������CO2��Һ̬ˮ���ų�������Ϊ889.6kJ/mol��2mol��1779.2kJ��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��HCl��NaOH��Ӧ���к��ȡ�H����57��3kJ/mol����H2SO4��Ca(OH)2��Ӧ���к���Ϊ2������57��3�� kJ/mol |
| B��CO(g)��ȼ������283��0 kJ/mol�����ʾCOȼ���ȵ��Ȼ�ѧ����ʽΪCO(g)��1/2O2(g)===CO2 (g)����H����283��0 kJ/mol |
| C����Ҫ���Ȳ��ܷ����ķ�Ӧһ�������ȷ�Ӧ |
| D��1 mol��ȼ��������̬�����������ų�����������ǵ�ȼ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
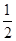 O2(g) ��H����266 kJ��mol��1
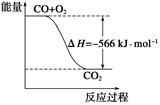
O2(g) ��H����266 kJ��mol��1| A��CO��ȼ����Ϊ283 kJ |
| B����ͼ�ɱ�ʾ��CO����CO2�ķ�Ӧ���̺�������ϵ |
| C��2Na2O2(s)��2CO2(s)===2Na2CO3(s)��O2(g)����H>��532kJ��mol��1 |
| D��CO(g)��Na2O2(s)��Ӧ�ų�549 kJ����ʱ������ת����Ϊ6��02��1023 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 2NH3Ϊ���ȷ�Ӧ���ɴ��ƶϳ� �� ��
2NH3Ϊ���ȷ�Ӧ���ɴ��ƶϳ� �� ��| A�������͵����ڳ��³�ѹ�£�����Ҫ�κ������Ϳ���Ѹ�ٷ�����Ӧ |
| B��3mol������1mol�������������������2mol��������������� |
| C������1molN��N����3molH��H�����յ�����С���γ�6molN��H�����ų������� |
| D�������͵����Ǹ��ܼ����ȶ������ʣ������ǵ����ҷdz��ȶ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������ʵ�����CH4�μӷ�Ӧ����Ӧ�٢�ת�Ƶĵ�������ͬ |
| B��CH4(g)+4NO2(g)=4NO(g)+CO2(g)+2H2O(g)��H=��530 kJ/mol |
| C��0.2 mol CH4��ԭNO2��N2��������H2O(g)�ų�������Ϊ164.6 kJ |
| D������4.48 L(��״��)CH4��ԭNO2��N2������H2O(g)������������ת�Ƶ���1.60 mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ����һ | CO(g) +2H2(g)  CH3OH(g) CH3OH(g) |
| ������ | CO2(g) +3H2(g)  CH3OH(g) +H2O(g) CH3OH(g) +H2O(g) |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������������ˮ��Ӧ | B����ˮ��ϡ����ķ�Ӧ |
| C�����ȵ�̿��CO2��Ӧ | D��Ba(OH)2��8H2O��NH4Cl �ķ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���


 MnO4-+
MnO4-+  H2O2+
H2O2+  _________=Mn2++
_________=Mn2++ O2��+
O2��+ ___��
___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
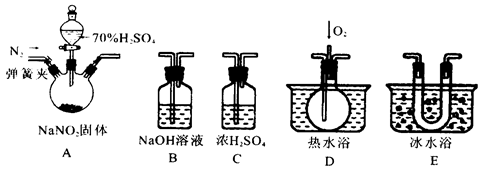
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com