
(6��)���з�Ӧ��CO(g)+ H2O(g) CO2(g)+ H2(g) ����H<0����850��ʱ��ƽ�ⳣ��K=1��
CO2(g)+ H2(g) ����H<0����850��ʱ��ƽ�ⳣ��K=1��
��1���������¶ȵ�950��ʱ���ﵽƽ��ʱK_____1������ڡ�����С�ڡ����ڡ���
��2��850��ʱ������һ�ݻ��ɱ���ܱ�������ͬʱ���� 1.0 mol CO��3.0molH2O��1.0mol CO2 �� x molH2����
�ٵ�x=5.0ʱ������ƽ����_______��������Ӧ���淴Ӧ��������С�
����Ҫʹ������Ӧ��ʼʱ������Ӧ������У���xӦ�����������________��
 ����С״Ԫ��������������ϵ�д�
����С״Ԫ��������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(9��)���з�Ӧ��mA(g)+nB(g)pC(g)���ﵽƽ��������¶�ʱ��B��ת���ʱ����Сѹǿʱ�������ϵ��C����������Ҳ��С����:
(1)�÷�Ӧ���淴ӦΪ_________�ȷ�Ӧ����m+n_________p(�������=��������)��
(2)��ѹʱ��A����������_________��(�������С�����䡱����ͬ)
(3)������B(�������)����A��ת����_________��B��ת����_________��
(4)�������¶ȣ���ƽ��ʱB��C��Ũ��֮��![]() ��______ ___��
��______ ___��
(5)�����������ƽ��ʱ��������������ʵ���____ _____��
(6)��B����ɫ���ʣ�A��C����ɫ�������C(�������)ʱ�������ɫ______ _����ά��������ѹǿ���䣬��������ʱ���������ɫ____ ___(��������dz�����䡱)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(6��) ����A��B��C��D��E��F��G�������ʣ���֪��A��B��C��D�����壬A���ܶ���С�����壬B��ͨ������³ʻ���ɫ������������ֱ�ͨ��������������Һ�У�ͨ��B��D����ʱ�������ְ�ɫ������������A������B�а�����ȼ�գ�������ɫ�Ļ��沢����D������ɫ�̼�����ζ����Cͨ�����ʯ��ˮʱ����ǡ�EΪ�����Ľ������ʣ�����D��ˮ��Һ������Ӧ����A��F��F��ͨ��B������Եõ�G
��1��D��F�Ļ�ѧʽ�ֱ�Ϊ��
D______________________ F_____________________
(2) C�ĵ���ʽΪ ��
(3) B��F����G�����ӷ���ʽΪ________________________________
(4) C�����ʯ��ˮ��Ӧ�����ӷ���ʽΪ ��
��5��ʵ������KSCN����G�������ӣ�������________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(6��)���п��淴ӦA(��)��B(��) 3C(��)����ͼ�мס��ҡ����ֱ��ʾ�ڲ�ͬ�������£�������C�ڷ�Ӧ������еİٷֺ���(C%)�ͷ�Ӧʱ��Ĺ�ϵ��
��1������ͼ���������߷ֱ��ʾ�д���������ʱ��������� �����DZ�ʾ�д���ʱ�������
��2������ͼ�е�a���߱�ʾ200���b���߱�ʾ100��ʱ���������˿��淴Ӧ������Ӧ�� �ȷ�Ӧ��
��3������ͼ���������߷ֱ��ʾ��ͬѹǿ�µ�������� �����DZ�ʾѹǿ�ϴ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ�ϱ�У���߶�9�·�������ѧ�Ծ� ���ͣ������
(9��)���з�Ӧ��mA(g)+nB(g) pC(g)���ﵽƽ��������¶�ʱ��B��ת���ʱ����Сѹǿʱ�������ϵ��C����������Ҳ��С����:
pC(g)���ﵽƽ��������¶�ʱ��B��ת���ʱ����Сѹǿʱ�������ϵ��C����������Ҳ��С����:
(1)�÷�Ӧ���淴ӦΪ_________�ȷ�Ӧ����m+n_________p(�������=��������)��
(2)��ѹʱ��A����������_________��(�������С�����䡱����ͬ)
(3)������B(�������)����A��ת����_________��B��ת����_________��
(4)�������¶ȣ���ƽ��ʱB��C��Ũ��֮��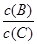 ��______ ___��
��______ ___��
(5)�����������ƽ��ʱ��������������ʵ���____ _____��
(6)��B����ɫ���ʣ�A��C����ɫ�������C(�������)ʱ�������ɫ______ _����ά��������ѹǿ���䣬��������ʱ���������ɫ____ ___(��������dz�����䡱)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��㶫ʡ��ݸ�и߶��ڶ�ѧ����ĩ���Ի�ѧB�� ���ͣ������
(6��)���п��淴ӦA(��)��B(��)  3C(��)����ͼ�мס��ҡ����ֱ��ʾ�ڲ�ͬ�������£�������C�ڷ�Ӧ������еİٷֺ���(C%)�ͷ�Ӧʱ��Ĺ�ϵ��
3C(��)����ͼ�мס��ҡ����ֱ��ʾ�ڲ�ͬ�������£�������C�ڷ�Ӧ������еİٷֺ���(C%)�ͷ�Ӧʱ��Ĺ�ϵ��



��1������ͼ���������߷ֱ��ʾ�д���������ʱ��������� �����DZ�ʾ�д���ʱ�������
��2������ͼ�е�a���߱�ʾ200���b���߱�ʾ100��ʱ���������˿��淴Ӧ������Ӧ�� �ȷ�Ӧ��
��3������ͼ���������߷ֱ��ʾ��ͬѹǿ�µ�������� �����DZ�ʾѹǿ�ϴ�������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com