
| kaC(CH3COOH) |
| Ka��C(CH3COOH) |
| C(CH3COO-) |
| kaC(CH3COOH) |
| 1.75��10-5��0.10 |
| 1.75 |
| kaC(CH3COOH) |
| 1.75��10-5��0.0010 |
| 1.75 |
| 1.75 |
| 1.75 |
| Ka��C(CH3COOH) |
| C(CH3COO-) |
| 1.75��10-5��(0.10-x) |
| 1.0 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| �� |
| �� |
 ����дһ�֣�
����дһ�֣� ����дһ�֣�
����дһ�֣�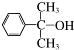 Ҳ����������Ʒ�Ӧ�۵ķ�Ӧ�����л���������Ľṹ��ʽ��
Ҳ����������Ʒ�Ӧ�۵ķ�Ӧ�����л���������Ľṹ��ʽ��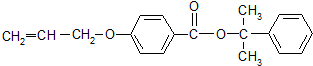

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
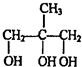

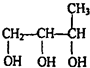

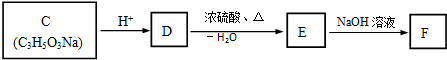
| һ������ |
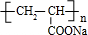
| һ������ |
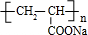
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� |










�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��ɽ��ʡ��У�������Ĵ��������ۻ�ѧ�Ծ��������棩 ���ͣ������
�״�ȼ�Ϸ�Ϊ�״����ͺͼ״����͡���ҵ�Ϻϳɼ״��ķ����ܶࡣ
��1��һ�������·�����Ӧ��
��CO2��g�� +3H2��g�� ��CH3OH��g��+H2O��g�� ? ��H1????
��2CO��g�� +O2��g�� ��2CO2��g��?? ��H2
��2H2��g��+O2��g�� ��2H2O��g��????????? ��H3
��CO��g�� + 2H2��g��  CH3OH��g��������H��??????????????? ��
CH3OH��g��������H��??????????????? ��
��2�����ݻ�Ϊ2L���ܱ������н��з�Ӧ�� CO��g��+2H2��g�� CH3OH��g�� �������������䣬��300����500��ʱ�����ʵ���n��CH3OH�� �뷴Ӧʱ��t�ı仯������ͼ��ʾ���÷�Ӧ����H???? 0 ����>��<��=����
CH3OH��g�� �������������䣬��300����500��ʱ�����ʵ���n��CH3OH�� �뷴Ӧʱ��t�ı仯������ͼ��ʾ���÷�Ӧ����H???? 0 ����>��<��=����

��3����Ҫ��״��IJ��ʣ��ɲ�ȡ�Ĵ�ʩ��____________������ĸ����
A�����������
B�������¶�
C�������¶�
D��ʹ�ú��ʵĴ���
E�����״��ӻ����ϵ�з������
��4��CH4��H2O�ڴ������淢����ӦCH4+H2O CO+3H2��T��ʱ����1 L�ܱ�������Ͷ��1 mol CH4��1 mol H2O��g����5Сʱ���÷�Ӧ��ϵ�ﵽƽ��״̬����ʱCH4��ת����Ϊ50% ��������¶��µ�ƽ�ⳣ��???? ���������С�������λ��������
CO+3H2��T��ʱ����1 L�ܱ�������Ͷ��1 mol CH4��1 mol H2O��g����5Сʱ���÷�Ӧ��ϵ�ﵽƽ��״̬����ʱCH4��ת����Ϊ50% ��������¶��µ�ƽ�ⳣ��???? ���������С�������λ��������
��5���Լ״�Ϊȼ�ϵ����͵�أ���ɱ�����������Ϊȼ�ϵĴ�ͳȼ�ϵ�أ�Ŀǰ�õ��㷺���о�����ͼ��Ŀǰ�о��϶��һ�����������ȼ�ϵ�ع���ԭ��ʾ��ͼ���ش��������⣺

��B���ĵ缫��ӦʽΪ????????????????????? ��
�����ø�ȼ�ϵ������Դ����ʯī���缫�������ͭ��Һ������·��ת��1mole- ʱ��ʵ�������ĵļ״��������������ϴ���ԭ����????????????????????? ��
��6��25��ʱ������Ƶ�Ksp=4.0��10-8,̼��Ƶ�Ksp=2.5��10-9����20ml̼��Ƶı�����Һ����μ���8.0��10-4 mol��L-1�IJ������Һ20ml���ܷ��������?????? ����������������������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com