
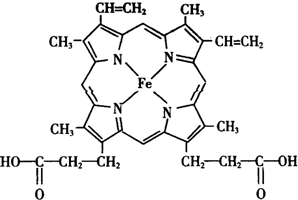
 �������Ͷ���ʹ��������������Ԫ�ص��о���Ȼ�ȶȲ�������ش�
�������Ͷ���ʹ��������������Ԫ�ص��о���Ȼ�ȶȲ�������ش� ����������ЦҼ��ͦм��ĸ�����Ϊ9��1��
����������ЦҼ��ͦм��ĸ�����Ϊ9��1��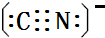 ���������廥Ϊ�ȵ����������N2��CO����д���֣��ѧʽ����
���������廥Ϊ�ȵ����������N2��CO����д���֣��ѧʽ���� ���� ��1�����ĵ����Ų�ʽΪ1s22s22p63s23p63d64s2��L�ܲ�Ϊ2s22p6����8�ֲ�ͬ�˶�״̬�ĵ��ӣ���̬��ԭ�ӵ���Χ�����Ų�ʽΪ3d64s2��
��2������ÿ��Nԭ�Ӻ��еĦ� ��������µ��Ӷ���֮���ж����ӻ���ʽ��Ѫ������Nԭ���еĺ���3���� ����һ���µ��Ӷԣ�����sp3�ӻ����еĺ���3���� ��������sp2 �ӻ���ʽ��N��Fe֮����ڵ�������Ǽ��Լ�����λ�����ʰ��ᣨ ��������Ϊ�Ҽ���˫����һ���Ҽ�������Ϊ�м���
��������Ϊ�Ҽ���˫����һ���Ҽ�������Ϊ�м���
��3������=$\frac{m}{V}$����̯�����㣻
��4����Ӧ�����ӷ���ʽΪ 3Fe2++2[Fe��CN��6]3-=Fe3[Fe��CN��6]2����������ΪCN-�����ĵ���ʽΪ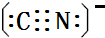 ���ȵ�����Ϊ��������ȣ�ԭ����Ҳ��ȣ�
���ȵ�����Ϊ��������ȣ�ԭ����Ҳ��ȣ�
��� �⣺��1�����ĵ����Ų�ʽΪ1s22s22p63s23p63d64s2��L�ܲ�Ϊ2s22p6����8�ֲ�ͬ�˶�״̬�ĵ��ӣ���̬��ԭ�ӵ���Χ�����Ų�ʽΪ3d64s2���ʴ�Ϊ��8��3d64s2��
��2������ÿ��Nԭ�Ӻ��еĦ� ��������µ��Ӷ���֮���ж����ӻ���ʽ��Ѫ������Nԭ���еĺ���3���� ����һ���µ��Ӷԣ�����sp3�ӻ����еĺ���3���� ��������sp2 �ӻ���ʽ��N��Fe֮����ڵ�������Ǽ��Լ�����λ�����ʰ��ᣨ ����������ЦҼ��ͦм��ĸ�����Ϊ9��1���ʴ�Ϊ��sp2��sp3�����Լ�����λ����9��1��
����������ЦҼ��ͦм��ĸ�����Ϊ9��1���ʴ�Ϊ��sp2��sp3�����Լ�����λ����9��1��
��3�����ݾ����е���λ�����һ�������к���2������1pm=10-10cm���ɦ�=$\frac{m}{V}$=$\frac{n•M}{{N}_{A}•��a��1{0}^{-10}��^{3}}$=$\frac{2��56}{{N}_{A}•{a}^{3}•1{0}^{-30}}$=$\frac{112}{{N}_{A}•{a}^{3}•1{0}^{-30}}$g/cm3���ʴ�Ϊ��$\frac{112}{{N}_{A}•{a}^{3}•1{0}^{-30}}$��
��4�����軯�أ�K3[Fe��CN��6]���׳Ƴ�Ѫ�Σ������ڼ���Fe2+����Ӧ�����ӷ���ʽΪ 3Fe2++2[Fe��CN��6]3-=Fe3[Fe��CN��6]2����������ΪCN-�����ĵ���ʽΪ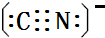 ���ȵ�����Ϊ��������ȣ�ԭ����Ҳ��ȣ��������廥Ϊ�ȵ����������N2��CO���ʴ�Ϊ��3Fe2++2[Fe��CN��6]3-=Fe3[Fe��CN��6]2��
���ȵ�����Ϊ��������ȣ�ԭ����Ҳ��ȣ��������廥Ϊ�ȵ����������N2��CO���ʴ�Ϊ��3Fe2++2[Fe��CN��6]3-=Fe3[Fe��CN��6]2��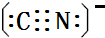 ��N2��CO��
��N2��CO��
���� ���⿼������Χ�����Ų�ʽ���ӻ����͡�����ʽ���ṹʽ����̯�����㾧�����ȵ�����Ȼ�������ۺ��Խ�ǿ���ѶȽϴ�


 ��Уͨ��֤��Ч��ҵϵ�д�
��Уͨ��֤��Ч��ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ع���ʱ��ZnΪ������������PH��� | |
| B�� | ��ظ����ĵ缫��ӦʽΪ��2MnO2��s��+H2O��l��+2e-�TMn2O3��s��+2OH-��aq�� | |
| C�� | ��ع���ʱ���������������� | |
| D�� | ���������������Һ��������Ǩ�Ƶ���ص����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | HPO32-���ܵ���Ҳ��ˮ�� | |
| B�� | Na2HPO3��Һһ���ʼ��� | |
| C�� | H3PO3���л�ԭ�� | |
| D�� | H3PO3����Һ�м������NaOH������Na3PO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
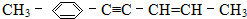 �������̼ԭ�������
�������̼ԭ������� ��
��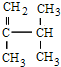 ϵͳ������������Ϊ2��3-����-1-��ϩ��
ϵͳ������������Ϊ2��3-����-1-��ϩ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
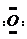 ]2-��CO2�ĽṹʽΪO=C=O����þͬ���ڡ����Ӱ뾶��С��Ԫ�أ���ԭ�������ĵ����Ų�ʽΪ3s23p1������������ߵĵ�����1����
]2-��CO2�ĽṹʽΪO=C=O����þͬ���ڡ����Ӱ뾶��С��Ԫ�أ���ԭ�������ĵ����Ų�ʽΪ3s23p1������������ߵĵ�����1�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��NH3������Nԭ�ӵ��ӻ���ʽΪSP3�ӻ���NH3���ӵĿռ����幹���������ͣ�
��NH3������Nԭ�ӵ��ӻ���ʽΪSP3�ӻ���NH3���ӵĿռ����幹���������ͣ�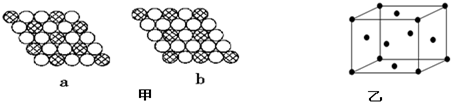
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 100��ʱ��0.001mol•L-1��NaOH��ҺpH=11 | |
| B�� | ����Һ��c��H+����c��OH-����ȣ���Һ�϶������� | |
| C�� | 25�棬0.001mol/LH2SO4��Һ�У�ˮ�������c��OH-��Ϊ10-11mol/L | |
| D�� | NaCl��Һ��CH3COONH4��Һ�������ԣ�����Һ��ˮ�ĵ���̶���ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ̼��������Ȼ���й㷺���ڣ�
̼��������Ȼ���й㷺���ڣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
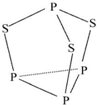 ��������������������Ħ���棬���ӽṹ��ͼ��ʾ�������й���������˵������ȷ���ǣ�������
��������������������Ħ���棬���ӽṹ��ͼ��ʾ�������й���������˵������ȷ���ǣ�������| A�� | ����������Ԫ�صĻ��ϼ�Ϊ+3 | |
| B�� | �����ʷ����в����Ǽ��Թ��ۼ� | |
| C�� | �����ʵ��ۡ��е��P4�� | |
| D�� | ������22 g����ԭ�ӵ���ĿԼΪ1.806��1023 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com