
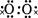
��D��Һ�����ˮ�пɵõ���FΪ��ɢ�ʵĺ��ɫ���塣��ش��������⣺
(1)���ɫ������F����ֱ����С�ķ�Χ��________________��
(2)A��B��H�Ļ�ѧʽ��A________��B________��H________��
(3)��H2O2���ӵĵ���ʽ��________________��
��д��C��������Һ��˫��ˮ��Ӧ�����ӷ���ʽ��
_____________________________________________________________________��
(4)д������E�������ӵ�ʵ�鷽��������___________________________________________________________________
_____________________________________________________________________��
(5)��C��Һ�м�����C�����ʵ�����Na2O2��ǡ��ʹCת��ΪF��д���÷�Ӧ�����ӷ���ʽ��
_____________________________________________________________________��
��1��1 nm��100 nm
��2��Fe FeS H2SO4��ϡ��
��3����H![]() H
H
��2Fe2++H2O2+2H+==2Fe3++2H2O
��4��ȡ����E���Թ��У��ý�ͷ�ιܵ���NaOH��Һ�������Թܣ��ɹ۲쵽�Թܿڴ�ʪ��ĺ�ɫʯ����ֽ�������������������𰸣�
��5��4Fe2++4Na2O2+6H2O==4Fe��OH��3��+O2��+8Na+
������A�ܺ�ϡH2SO4��Ӧ����һ�ֻ��ý�����FΪ���ɫ���壬��ȷ��ΪFe(OH)3����AΪFe����һ��ȷ��BΪFeS��CΪFeSO4��DΪFe2(SO4)3��EΪ(NH4)2SO4��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
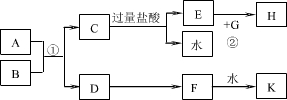
��ѧ��ѧ�м��ֳ������ʵ�ת����ϵ��ͼ��ʾ(ͼ�в��ַ�Ӧ��������P��Ӧ����δ�г�)��
 |
��֪��A��B��C��D��E�ǵ��ʣ�������ǵ�Ԫ�ص�ԭ����������Ϊa��b��c��d��e����3(a + b) = 2(a + c) = 3(d ��a)��X��Y��Z��M��N��W��H��K�ǻ��������X��B��C�Ļ��ϲ����ˮ��Һ����ش��������⣺
��1���õ���ʽ��ʾX�����ʵ��γɹ��̣�_______________�����C��Ԫ�ص�ԭ�ӽṹʾ��ͼ��_____ ��
��2��д��B��������ȼ�����ɵIJ�����H2O��Ӧ�Ļ�ѧ����ʽ��_______________��
��3����ɵ���B��C��D������Ԫ�ؼ����ӵ����Ӱ뾶�ɴ�С��˳����__ _(�����ӷ��ű�ʾ)��
��4��д��K������İ�ˮ��Ӧ�Ļ�ѧ����ʽ ��д��M��ˮ��Һ�е���ķ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ��ѧ�м��ֳ������ʵ�ת����ϵ����ͼ��ʾ�����ַ�Ӧ������P��Ӧ��������ȥ����
��֪��B��C��DΪ�������ʣ�����CΪ���壬B��DΪ������FΪ������ǿ�K������Ϊ���壬Ŀ��ʹƷ����Һ��ɫ���� E ��Һ�����ˮ���Ƶ�һ�ֺ��ɫ���壻 J Ϊ����ɫ���塣��ش��������⣺
 |
( l ) B �Ļ�ѧʽΪ ��
д�� B �� F ��Ӧ�Ļ�ѧ����ʽ
( 2��ʵ���б��� G ��ҺʱҪ���� Ŀ����
( 3��Ϊʵ��JһD�ı仯����X�Ƿǽ������ʣ���X������ ��д��ѧʽ��; ��X�ǽ������ʣ���д�� J һ D ��Ӧ�Ļ�ѧ����ʽ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com