

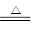 2BF3��+3CaSO4 +3H2O ��
2BF3��+3CaSO4 +3H2O �� 2BN+3H2O��
2BN+3H2O��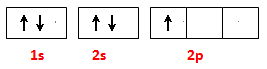 �� +3
�� +3  2BF3��+3CaSO4 +3H2O��B2O3+2NH3
2BF3��+3CaSO4 +3H2O��B2O3+2NH3 2BN+3H2O��
2BN+3H2O��

 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Na2O2�����е������Ӻ������� |
| B��NaHCO3�����е������Ӻ������� |
C��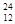 Mg2+�����е����Ӻ����� Mg2+�����е����Ӻ����� |
| D����������Һ�е����������������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
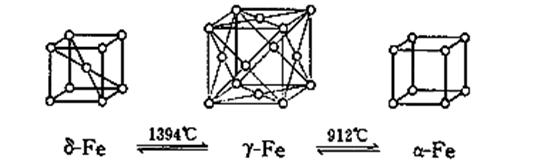
| A����-Fe��������������ԭ�Ӿ���������������ԭ����8�� |
| B����-Fe��������������ԭ�Ӿ���������������ԭ����12�� |
| C����-Fe�����߳���Ϊa cm����-Fe�����߳���Ϊb cm�����-Fe�ͦ�-Fe���־�����ܶȱ�Ϊb3��a3 |
| D���������ȵ�1500 ��ֱ�����ȴ�ͻ�����ȴ���õ��ľ���ṹ����ͬ������ѧ���ʼ�����ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��F2��Cl2��Br2��I2���ۡ��е������� |
| B��HF��HCl��HBr��HI�����ȶ������μ��� |
| C�����ʯ��Ӳ�ȡ��۵㡢�е㶼���ھ���� |
| D��NaF��NaCl��NaBr��NaI���۵����ν��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
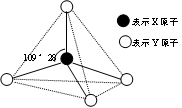
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��NaCl SiO2 CO2 Na�� | B��HI HBr HCl HF������ |
| C��NaCl KCl KBr KI | D��P4 N2 Br2 O2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ԭ�ӵĺ�������ڽ��������ж������ɵ��� |
| B��þ�ͺ�ͭ�͵�ԭ�Ӷѻ���ʽ�ռ���������� |
| C������ԭ���ڻ�ѧ�仯��ʧȥ�ĵ�����Խ�࣬�仹ԭ��Խǿ |
| D���¶����ߣ������ĵ����Խ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����Ӿ�����һ�����ڹ��ۼ� |
| B���ڢ�A���⻯������Է����������ӣ��۷е������ߣ�HF�е���� |
| C�����Ӿ������ۻ�ʱ�����Ӽ����ƻ��������Ӿ����ۻ�ʱ����ѧ�������ƻ� |
| D������״̬�µ���ľ���һ���ǽ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
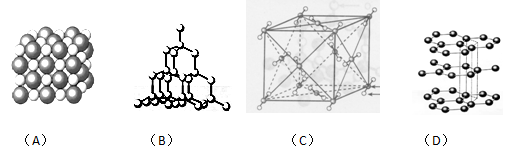
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com