
��10�֣�ijʵ��С����0��50 mol��L��1 NaOH��Һ��0��50 mol��L��1������Һ�����к��ȵIJⶨ��
������0��50 mol��L��1 NaOH��Һ
��1����ʵ���д�ԼҪʹ��245 mL NaOH��Һ��������Ҫ����NaOH����________g��
��2������ͼ��ѡ�����NaOH��������Ҫ������(����ĸ)��__________��
���� | ������ƽ(������) | С�ձ� | ����ǯ | ������ | ҩ�� | ��Ͳ |
���� |
|
|
|
|
|
|
��� | a | b | c | d | e | f |
�ⶨϡ�����ϡ���������к��ȵ�ʵ��װ������ͼ��ʾ��
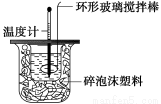
��1��д���÷�Ӧ���Ȼ�ѧ����ʽ(�к���Ϊ57��3 kJ��mol��1)��_________________________
________________________________________________________________________��
��2��ȡ50 mL NaOH��Һ��30 mL������Һ����ʵ�飬ʵ���������±���
������д�±��еĿհף�
ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | �¶Ȳ�ƽ��ֵ (t2��t1)/�� | ||
H2SO4 | NaOH | ƽ��ֵ | |||
1 | 26��2 | 26��0 | 26��1 | 30��1 |
|
2 | 27��0 | 27��4 | 27��2 | 31��2 | |
3 | 25��9 | 25��9 | 25��9 | 29��8 | |
4 | 26��4 | 26��2 | 26��3 | 30��4 | |
�ڽ�����Ϊ0��50 mol��L��1 NaOH��Һ��0��50 mol��L��1������Һ���ܶȶ���1 g��cm��3���кͺ�������Һ�ı�����c��4��18 J��g��1���棭1�����к��Ȧ�H��_______________________(ȡС�����һλ)��
������ʵ����ֵ�����57��3 kJ��mol��1��ƫ�����ƫ���ԭ�������(����ĸ)____________��
a��ʵ��װ�ñ��¡�����Ч����
b����ȡNaOH��Һ�����ʱ���Ӷ���
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
44����1��5��0��2��abe
��1��H2SO4(aq)��2NaOH(aq)===Na2SO4(aq)��2H2O(l)��H����114��6 kJ��mol��1
��2����4��0�ڣ�53��5 kJ��mol��1��acd
��������
�����������1��245ml����Һ����Ҫ����250ml����Һ������Ҫ�������250mL��������������Ƶ���������2������������ŵ�С�ձ��г��������õ���ƽ��С�ձ���Կ�ף�II��1���к�����ָ����1molH2O�ų���������������2molH2Oʱ�ų�������Ҳ�ӱ�����2������ֹ�¶ȼ�ȥ��ʼ�¶�ƽ��ֵ�����ÿ�ε��¶Ȳ�����Ĵε�ƽ��ֵ���ڱ����ݳ�����Һ�����������������������1molˮ�ų������������kJ�����к��ȣ���bѡ����ȡ��������ʱ���Ӷ�����ʹ��ȡ��������������ų��������࣬��b����ac������������ʧ������ȷ��d���¶ȼ��ϸ��ŵ��������ƺ����ᷴӦ�ˣ��ⲿ��������ʧ�ˣ���ѡacd��
���㣺�к��ȵĶ��塢����������Һ�����ơ��к��ȵIJ�������������֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013-2014������ʡ�߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
MnO2��һЩ���ʻ���;��ͼ������˵����ȷ���� ( )
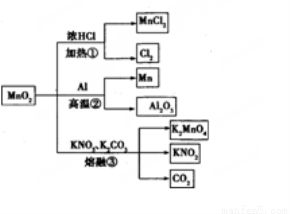
A���١��ڡ���������Ӧ��MnO2����������
B������MnO2��2 L 10 mol/LHCl���ȣ�������5 mol C12
C����Ӧ��������1 mol Al2O3����Ӧ������ת��12 mol����
D����Ӧ����K2CO3��KNO3�Ļ�ѧ��������Ϊ1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014������ʡ��һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
ͬλ��2H��15N��18O��34S�ȳ�������½����̬�����о�������˵������ȷ����
A��16O��18O��ͬһ�ֺ��� B�� 1H218O��Ħ������Ϊ20
C��34S��15N���ڵ����������9 D��2H�����������Ϊ0
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014������ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��Է�������Ϊ100������ȫȼ�պ�����CO2��H20�����ʵ���֮���Լ����ӽṹ�����ĸ�����ͬ���칹�����Ŀ�ֱ��ǣ� ��
A��6��7��2 B��6��7��3 C��7��8��3 D��7��8��4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014������ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��������6��̼ԭ�ӣ��������һ�����֧���������� �� ��
A��2�֣� B.4�� C.8�� D��12��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014������ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪��
2CO(g)��O2(g)===2CO2(g)����H����566 kJ��mol��1
Na2O2(s)��CO2(g)===Na2CO3(s)��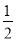 O2(g) ��H����266 kJ��mol��1
O2(g) ��H����266 kJ��mol��1
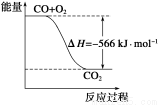
���������Ȼ�ѧ����ʽ�жϣ�����˵����ȷ����
A��CO��ȼ����Ϊ283 kJ
B����ͼ�ɱ�ʾ��CO����CO2�ķ�Ӧ���̺�������ϵ
C��2Na2O2(s)��2CO2(s)===2Na2CO3(s)��O2(g)����H>��532kJ��mol��1
D��CO(g)��Na2O2(s)��Ӧ�ų�549 kJ����ʱ������ת����Ϊ6��02��1023
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014������ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪ͬ����X��Y��Z����Ԫ�ص�����������Ӧˮ����������ǿ������˳��Ϊ��
HXO4��H2YO4��H3ZO4���������ж�����ȷ����
A��Ԫ�طǽ�����X��Y��Z��˳�����
B��ԭ�ӵĵ���������X��Y��Z��˳����ǿ
C����̬�⻯����ȶ���X��Y��Z��˳����ǿ
D�����ʵ�������X��Y��Z��˳����ǿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014������ʡ˫Ѽɽ�и߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
������Ũ�ȶ�Ϊ0.1 mol/L��������Һ�У����루��ͨ�룩ij���ʺ�����Ӧ�Ⱥ�˳����ȷ����
A���ں�Fe3+��Cu2+��H+����Һ�м���п�ۣ�Cu2+��Fe3+��H+
B���ں�I����SO32����Br������Һ�в���ͨ��������I����Br����SO32��
C���ں�Fe3+��H+��NH4+����Һ����μ����ռ���Һ��Fe3+��NH4+��H+
D���ں�AlO2����SO32����OH������Һ����μ�������������Һ��OH����AlO2����SO32��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014������ʡ˫Ѽɽ�и�һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��Ӧ3X(g)+Y(g) 2Z(g)+2W(g)��2L�ܱ������н��У�5min��Y������0.5mol����˷�Ӧ��ƽ�����ʦ�Ϊ( )
2Z(g)+2W(g)��2L�ܱ������н��У�5min��Y������0.5mol����˷�Ӧ��ƽ�����ʦ�Ϊ( )
A. ��(X)��0.05mol��L��1��min��1 B. ��(Y)�� 0.10mol��L��1��min��1
C. ��(Z)��0.10mol��L��1��min��1 D. ��(W)��0.05mol��L��1��s��1
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com