
������Ũ�ȶ�Ϊ0.1 mol/L��������Һ�У����루��ͨ�룩ij���ʺ�����Ӧ�Ⱥ�˳����ȷ����
A���ں�Fe3+��Cu2+��H+����Һ�м���п�ۣ�Cu2+��Fe3+��H+
B���ں�I����SO32����Br������Һ�в���ͨ��������I����Br����SO32��
C���ں�Fe3+��H+��NH4+����Һ����μ����ռ���Һ��Fe3+��NH4+��H+
D���ں�AlO2����SO32����OH������Һ����μ�������������Һ��OH����AlO2����SO32��
D
��������
���������A�������ԣ�Fe3+��Cu2+��H+������������ԭ��Ӧ���Ⱥ���ɣ�������Ӧ�Ⱥ�˳��Ϊ��Fe3+��Cu2+��H+����A����B����ԭ�ԣ�SO32-��I-��Br-������������ԭ��Ӧ���Ⱥ���ɣ�������Ӧ�Ⱥ�˳��Ϊ��SO32-��I-��Br-����B����C���ȶ��ԣ�ˮ������������һˮ�ϰ����ʷ�����Ӧ�Ⱥ�˳��Ϊ��H+��Fe3+��NH4+����C����D�����ԣ�OH-��AlO2-��SO32-���ʷ�����Ӧ�Ⱥ�˳��Ϊ��OH-��AlO2-��SO32-����D��ȷ����ѡD��
���㣺���������ԡ���ԭ��ǿ���Ƚϣ����ӷ�Ӧ����������


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013-2014������ʡ��һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
ͬһ���������Ԫ�ص�ԭ������֮����ܵ���
A��16 B��26 C��36 D��46
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014������ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��10�֣�ijʵ��С����0��50 mol��L��1 NaOH��Һ��0��50 mol��L��1������Һ�����к��ȵIJⶨ��
������0��50 mol��L��1 NaOH��Һ
��1����ʵ���д�ԼҪʹ��245 mL NaOH��Һ��������Ҫ����NaOH����________g��
��2������ͼ��ѡ�����NaOH��������Ҫ������(����ĸ)��__________��
���� | ������ƽ(������) | С�ձ� | ����ǯ | ������ | ҩ�� | ��Ͳ |
���� |
|
|
|
|
|
|
��� | a | b | c | d | e | f |
�ⶨϡ�����ϡ���������к��ȵ�ʵ��װ������ͼ��ʾ��
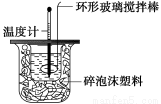
��1��д���÷�Ӧ���Ȼ�ѧ����ʽ(�к���Ϊ57��3 kJ��mol��1)��_________________________
________________________________________________________________________��
��2��ȡ50 mL NaOH��Һ��30 mL������Һ����ʵ�飬ʵ���������±���
������д�±��еĿհף�
ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | �¶Ȳ�ƽ��ֵ (t2��t1)/�� | ||
H2SO4 | NaOH | ƽ��ֵ | |||
1 | 26��2 | 26��0 | 26��1 | 30��1 |
|
2 | 27��0 | 27��4 | 27��2 | 31��2 | |
3 | 25��9 | 25��9 | 25��9 | 29��8 | |
4 | 26��4 | 26��2 | 26��3 | 30��4 | |
�ڽ�����Ϊ0��50 mol��L��1 NaOH��Һ��0��50 mol��L��1������Һ���ܶȶ���1 g��cm��3���кͺ�������Һ�ı�����c��4��18 J��g��1���棭1�����к��Ȧ�H��_______________________(ȡС�����һλ)��
������ʵ����ֵ�����57��3 kJ��mol��1��ƫ�����ƫ���ԭ�������(����ĸ)____________��
a��ʵ��װ�ñ��¡�����Ч����
b����ȡNaOH��Һ�����ʱ���Ӷ���
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014������ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
Ԫ�����ڱ���һ�����ŵġ�Ԫ�ش��á�����Ԫ�ش��á���δ������������119��Ԫ�أ����ڡ�Ԫ�ش��á��а��ź����ġ����䡱
A����������0�� B���������ڵڢ�A��
C���ڰ����ڵڢ�A�� D���������ڵڢ�A��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014������ʡ˫Ѽɽ�и߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
��8�֣���100 mL��NaOH��Һ��ͨ��CO2��ַ�Ӧ���ڼ�ѹ�ͽϵ��¶��£�С�ĵؽ���Һ���ɣ��õ���ɫ����M��ͨ���CO2�����V(��״��)��M������W�Ĺ�ϵ����ͼ��ʾ���Խ���������⣺
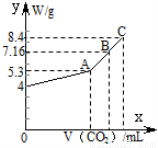
(1)A��ʱ����ɫ����M�Ļ�ѧʽΪ____________________��ͨ���CO2�����Ϊ________ mL (��״���£���ͬ)��
(2)C��ʱ����ɫ����M�Ļ�ѧʽΪ____________________��ͨ���CO2�����Ϊ________ mL��
(3)B��ʱM����ɳɷ�Ϊ________(�û�ѧʽ��ʾ)��ͨ���CO2�����Ϊ________ mL��
(4)��NaOH��Һ�����ʵ���Ũ��Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014������ʡ˫Ѽɽ�и߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�������ӷ���ʽ��ȷ����
A��0��01mol/L NH4Al��SO4��2��Һ��0��02 mol/LBa��OH��2��Һ�������ϲ���������
NH4++Al3++2SO42?+2Ba2++4OH?=2BaSO4��+ Al��OH��3��+NH3��H2O
B��NH4HCO3��Һ������Ba��OH��2��Һ��ϣ�HCO3����Ba2����OH����BaCO3����H2O
C����NaHCO3��Һ�м�������ij���ʯ��ˮ�����ְ�ɫ������ 2HCO3-+Ca2++2OH-=CaCO3��+CO32-+2H2O
D����FeCl3��Һ�м���Na2S��Һ����������2Fe3++3S2-+6H2O=2Fe��OH��3��+3H2S��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014������ʡ˫Ѽɽ�и߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
���и���������ָ����Һ���ܴ���������ǣ� ��
����ɫ��Һ�У�K+��H2PO4����SO42����PO43��
��pH=11����Һ�У�CO32����Na+��AlO2����NO3��
�ۼ���Al�ܷų�H2����Һ�У�Cl����HCO3����SO42����NH4+
��ͨ��CO2����Һ�У�Na+��Ba2+��Cl����Br��
���н϶�Fe2+����Һ�У� Na+��NH4+��[Fe(CN)6]3����CO32��
��������Һ�У�Na+��Al3+��NO3����I��
A���٢�B���ۢ�C���ڢ�D���ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014������ʡ˫Ѽɽ�и�һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
������̬����ɵĻ�����壬��ȫȼ�պ�õ�CO2��H2O�����ʵ������Ż�������ʵ����ı仯��ͼ��ʾ�������жԻ�������ж���ȷ����
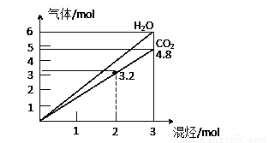
�ٿ�����C2H2
��һ����CH4
��һ����C3H8
��һ��û��C2H6
�ݿ�����C2H6
A���ڢݡ��� B���ڢ� C���ۢ� D���ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014����������һ�С���һ�й��ʺ���У���������¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�����£����и���������ָ����Һ��һ���ܴ����������
A������Mg�ܷų�H2����Һ��Na+��Al3+��Cl����SO42��
B�������£�c(H+)/c(OH��)=1010����Һ��Fe2+��K+��NO3����SO42��
C�����뱽������ɫ����Һ��NH4����K+��Cl����S2��
D��ʹ���ȳʺ�ɫ����Һ��NH4����Na����AlO2����HCO3��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com