����FeCl
3��ˮ��Һ��
����
����
������ԡ��������ԡ������ԡ�����ԭ����
Fe3++3H2O?Fe��OH��3+3H+��
Fe3++3H2O?Fe��OH��3+3H+��
�������ӷ���ʽ��ʾ����
ʵ��������FeCl
3��ˮ��Һʱ������FeCl
3���������ڽ�Ũ�������У�Ȼ����������ˮϡ�͵������Ũ�ȣ���
����
����
����ٽ����������ơ�����ˮ�⣻�����FeCl
3��Һ���ɣ����գ����õ�����Ҫ���������
Fe2O3
Fe2O3
��
����ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�
����0.10mol/L�ı�������ϴ��ʽ�ζ���2��3��
��ȡ������ע����ʽ�ζ�������0���̶�����2��3mL��
�۰�ʢ�б��������ʽ�ζ��̶ܹ��ã����ڵζ��ܼ���ʹ֮������Һ
�ܵ���Һ������0����0���̶����£����¶���
��ȡ20.00mL����NaOH��Һע��ྻ����ƿ�У�������2��3�μ�����Һ
�ް���ƿ���ڵζ��ܵ����棬�ñ�����ζ����յ㣬��¼�ζ��ܶ���
��ش��������⣺
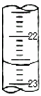
��1��ij�εζ�ʱ�ĵζ����е�Һ����ͼ��ʾ�������Ϊ
22.60
22.60
mL��
��2�������������ݣ�
| ����� |
����Һ�����mL�� |
�����������mL�� |
| �ζ�ǰ������mL�� |
��������mL�� |
| ��һ�� |
20.00 |
0.50 |
25.40 |
| �ڶ��� |
20.00 |
4.00 |
29.10 |
���������ռ���Һ��Ũ��Ϊ
0.1250
0.1250
mol/L��
��3������ʵ�������ʹʵ����ƫ�ߵ���
A��B��C��D
A��B��C��D
��
A����ƿ�ô���Һ��ϴ����ע�����Һ
B����ʽ�ζ���δ�ñ�Һ��ϴ����װ���Һ
C����ʽ�ζ��ܵζ�ǰ���Ӷ������ζ������Ӷ���
D����ʽ�ζ��ܵζ�ǰ�����ݣ��ζ��������ݣ�

 ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�
ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
 ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�
ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£� ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�
ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£� ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�
ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�