
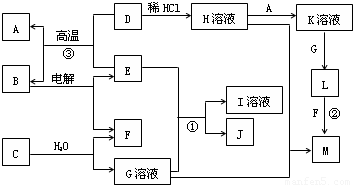
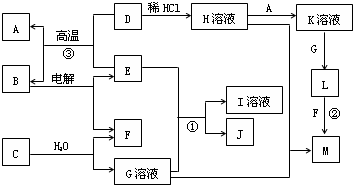
 H��Һ��H��Һ
H��Һ��H��Һ M��֪��HΪFeCl3����H
M��֪��HΪFeCl3����H K��Һ
K��Һ L
L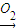 Fe��OH��3����LΪFe��OH��2��A�ǽ�������AΪFe��KΪFeCl2��
Fe��OH��3����LΪFe��OH��2��A�ǽ�������AΪFe��KΪFeCl2�� Fe+B���������ȷ�Ӧ��EΪAl��BΪAl2O3�����Al2O3����Al��O2������ת����ϵ���ɷ�Ӧ��Al��NaOH��Һ��Ӧ����NaAlO2��H2��JΪ���嵥�ʣ���JΪH2��IΪNaAlO2��
Fe+B���������ȷ�Ӧ��EΪAl��BΪAl2O3�����Al2O3����Al��O2������ת����ϵ���ɷ�Ӧ��Al��NaOH��Һ��Ӧ����NaAlO2��H2��JΪ���嵥�ʣ���JΪH2��IΪNaAlO2�� H��Һ��H��Һ
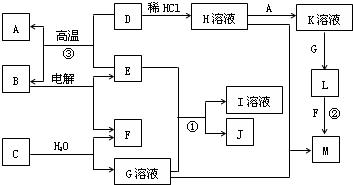
H��Һ��H��Һ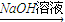 M��֪��HΪFeCl3����H
M��֪��HΪFeCl3����H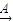 K��Һ
K��Һ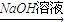 L
L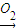 Fe��OH��3����LΪFe��OH��2��A�ǽ�������AΪFe��KΪFeCl2��
Fe��OH��3����LΪFe��OH��2��A�ǽ�������AΪFe��KΪFeCl2�� Fe+B���������ȷ�Ӧ��EΪAl��BΪAl2O3�����Al2O3����Al��O2������ת����ϵ���ɷ�Ӧ��Al��NaOH��Һ��Ӧ����NaAlO2��H2��JΪ���嵥�ʣ���JΪH2��IΪNaAlO2��
Fe+B���������ȷ�Ӧ��EΪAl��BΪAl2O3�����Al2O3����Al��O2������ת����ϵ���ɷ�Ӧ��Al��NaOH��Һ��Ӧ����NaAlO2��H2��JΪ���嵥�ʣ���JΪH2��IΪNaAlO2�� ��19.5gNa2O2�����ʵ���Ϊ
��19.5gNa2O2�����ʵ���Ϊ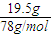 =0.25mol��Na2O2��ȫ��Ӧת�Ƶ������ʵ���Ϊ0.25mol×
=0.25mol��Na2O2��ȫ��Ӧת�Ƶ������ʵ���Ϊ0.25mol× ×2=0.25mol��
×2=0.25mol�� 2Fe+Al2O3��
2Fe+Al2O3�� 2Fe+Al2O3��
2Fe+Al2O3��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����˳һģ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com