
Ϊ�������÷Ϸ�����(����V2O5��VOSO4�������Բ���)��������Ա����������һ�����ӽ��������շ����¹��գ���Ҫ�������£�

���ֺ���������ˮ�е��ܽ������£�
���� | VOSO4 | V2O5 | NH4VO3 | (VO2)2SO4 |
�ܽ��� | ���� | ���� | ���� | ���� |
�ش��������⣺
��1����ҵ��V2O5ұ���������������ȼ������÷�Ӧ��������Ϊ______________________��
��2����Һ�к�������Ҫ�ɷ�Ϊ_____________(�ѧʽ)��
��3���ù����з�Ӧ�۵ij�����(�ֳƳ�����)�ǻ��շ��Ĺؼ�֮һ���ò���Ӧ�����ӷ���ʽ______________________�������ʵĸߵͳ�����ҺpHӰ���⣬����Ҫ�����Ȼ��ϵ��(NH4Cl������������Һ��V2O5��������)���¶ȣ�������ͼ�ж���ѿ����Ȼ��ϵ�����¶�Ϊ______��_____�档

��4���������ữ��H2C2O4��Һ�ζ�(VO2)2SO4��Һ���Բⶨ��Ӧ�ں���Һ�к���������Ӧ����ʽΪ��2VO2++H2C2O4+2H+=2VOn++2CO2��+mH2O������n��m�ֱ�Ϊ______��_____��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�����ʡ�߶����������ƻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
��t��ʱ����a gNH3��ȫ�ܽ���ˮ���õ�VmL��Һ���������Һ���ܶ�Ϊ�� g•cm-3����������Ϊ�أ����к�NH4+�����ʵ���Ϊb mol������������ȷ����( )
A�����ʵ�����������=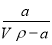 ��100%
��100%
B�����ʵ����ʵ���Ũ��c=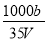 mol/L
mol/L
C����Һ��c(OH-)=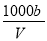 mol/L+ c(H+)
mol/L+ c(H+)
D��������Һ���ټ���VmLˮ��������Һ��������������0.5��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�������������и߶������л�ѧ�Ծ��������棩 ���ͣ�ѡ����
ij�л�����ҩ���������м��壬��ṹ��ʽ����ͼ��

�����й���������ȷ����( )
A�����л�����Ũ��ˮ�ɷ���ȡ����Ӧ
B�����л�����Ũ�����Ϲ��ȿɷ�����ȥ��Ӧ
C��1mol���л���������NaOH��Һ��Ӧ�������4molNaOH
D�����л��ᆳ��������������������ͭ����Һ��������ש��ɫ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�콭��ʡ����5��ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ������
�о����֣�NOx��SO2����������Ҫ�ɷ֡�
(һ)NOx��Ҫ��Դ������β����
��֪��N2(g)+O2(g) 2NO(g)��H=+180.50kJ•mol-1
2NO(g)��H=+180.50kJ•mol-1
2CO(g)+O2(g) CO2(g)��H=-566.00kJ•mol-1
CO2(g)��H=-566.00kJ•mol-1
��1��Ϊ�˼��������Ⱦ���������������β�������ܿڲ��ô�����NO��COת��������Ⱦ����� �����ѭ����д���÷�Ӧ���Ȼ�ѧ����ʽ___________________________��
��2��T��ʱ���������ʵ�����NO��CO�����ݻ�Ϊ2L���ܱ������У������¶Ⱥ�������䣬��Ӧ����(0-15min)��NO�����ʵ�����ʱ��仯����ͼ��ʾ��

��T��ʱ�û�ѧ��Ӧ��ƽ�ⳣ��K=____________��ƽ��ʱ�������¶Ȳ��䣬���������г���CO��N2��0.8mol��ƽ�⽫__________�ƶ���(����������ҡ�����)
��ͼ1��a��b�ֱ��ʾ��һ���¶��£�ʹ��������ͬ���������ͬ�Ĵ���ʱ���ﵽƽ�������n(NO)�ı仯���ߣ����б�ʾ����������ϴ��������_______(�a����b��)
��15minʱ�����ı���練Ӧ����������n(NO)������ͼ��ʾ�ı仯����ı������������____________��
(��)SO2��Ҫ��Դ��ú��ȼ�գ�ȼú��������������Ǽ��ٴ����к�������Ⱦ�Ĺؽ���
��3���ô�����Һ����SO2�ɽ���ת��ΪHSO3-���÷�Ӧ�����ӷ���ʽ��________________��
��4����ͼ��ʾ�ĵ��װ�D�ɽ������е�NO��SO2�ֱ�ת��ΪNH4+��SO42-��

��д������A�Ļ�ѧʽ_________�������ĵ缫��Ӧʽ��________________��
�ڸõ�ⷴӦ�Ļ�ѧ����ʽΪ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016�콭��ʡ����5��ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
��NA��ʾ������������ֵ������˵����ȷ����
A��1mol•L-1Na2CO3��Һ�У���CO32-��ĿС��NA
B��1mol FeCl3��ȫת��Ϊ����������������н���������ĿΪNA
C��25��ʱ��1LpH=12��Ba(OH)2��Һ�к��е�OH-��ĿΪ0.02NA
D��1mol���ͱ�����Ļ������ȫȼ��ʱ����O2�ķ�����Ϊ7.5NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�꽭������һ�и߶��µڶ��ζο���ѧ�Ծ��������棩 ���ͣ�ѡ����
����ʵ�����������������ƫ�ߵ���
������֪Ũ�ȵ�����ζ�δ֪Ũ�ȵ�NaOH��Һʱ����ʽ�ζ���δ�ñ�Һ��ϴ��
������Ͳ��ȡ5.0mL��Һʱ�����Ӷ�����
������һ�����ʵ���Ũ�ȵ�������Һ������ʱ��������ƿ�Ŀ̶��ߣ�
������֪Ũ�ȵ�����ζ�δ֪Ũ�ȵ�NaOH��Һʱ���ζ�ǰƽ�Ӷ������ζ��յ����Ӷ�����
A���ۢ� B���ڢ� C���٢� D���٢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�꽭������һ�и߶��µڶ��ζο���ѧ�Ծ��������棩 ���ͣ�ѡ����
һ��-C3H7��һ��-C3H7Oȡ�������ϵ���ԭ�ӣ��γɵ��л�������������Ʒ�Ӧ��ͬ���칹����(�����������칹)
A��18�� B��24�� C��30�� D��36��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�������и߶������л�ѧ�Ծ��������棩 ���ͣ�ѡ����
ԭ��Cr2O72-+CH3CH2OH+H++H2O [Cr(H2O)6]3++CH3COOH(δ��ƽ)�����ڼ��˾���Ƿ�ƺ��ʻ������˵����ȷ����
[Cr(H2O)6]3++CH3COOH(δ��ƽ)�����ڼ��˾���Ƿ�ƺ��ʻ������˵����ȷ����
A������1molCH3CH2OHʱת�Ƶ��ӵ����ʵ���Ϊ4mol
B��1mol/LCH3COOH��Һ�к��Цļ�����ĿΪ7NA
C��H2F+��NH2-��H2S��CH4����H2O��Ϊ�ȵ�����
D���������[Cr(H2O)6]3+�У�H��Oԭ������������Cr3+�γ���λ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016��ӱ�ʡ����ģ��Ѻ�����ۻ�ѧ�Ծ��������棩 ���ͣ������
�״���һ������ȼ�ϣ��ڹ�ҵ�ϳ���CO��H2�ϳɼ״�����Ӧ����ʽΪCO(g)+2H2(g)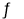 CH3OH(g)��
CH3OH(g)��
��֪��
��CO(g)��1/2O2(g)��CO2(g) ��H1����283.0kJ/mol
��H2(g)��1/2O2(g)��H2O(g) ��H2����241.8kJ/mol
��CH3OH(g)��3/2O2(g)��CO2(g)��2H2O(g) ��H3����192.2kJ/mol
�ش��������⣺
��1������CO(g)+2H2(g)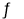 CH3OH(g)�ķ�Ӧ�ȡ�H4=____________��
CH3OH(g)�ķ�Ӧ�ȡ�H4=____________��
��2�����ھ��ȡ����ݵ��ܱ������г���1 mol CO��2 mol H2������CO(g)+2H2(g)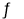 CH3OH(g)��Ӧ������ʾ��ͼ��ȷ����˵����Ӧ�ڽ��е�t1ʱ��Ϊƽ��״̬����________(��ѡ����ĸ)��
CH3OH(g)��Ӧ������ʾ��ͼ��ȷ����˵����Ӧ�ڽ��е�t1ʱ��Ϊƽ��״̬����________(��ѡ����ĸ)��
��3��T1��ʱ����һ�����Ϊ5 L�ĺ��������г���1 mol CO��2 mol H2������5 min�ﵽƽ�⣬CO��ת����Ϊ0.8����5 min����H2��ʾ�ķ�Ӧ����Ϊv(H2)=_______��T1��ʱ������һ���������ܱ�������Ҳ����1 mol CO��2 mol H2���ﵽƽ��ʱCO��ת����Ϊ0.7��������������____5 L(�>����<����=��)��T1��ʱ��CO(g)+2H2(g) CH3OH(g)��ƽ�ⳣ��K=______________��
CH3OH(g)��ƽ�ⳣ��K=______________��
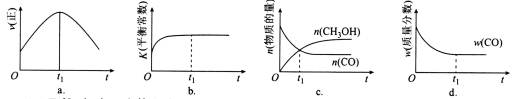
��4����T1��ʱ�������Ϊ5 L�ĺ��������г���һ������H2��CO����Ӧ�ﵽƽ��ʱCH3OH�����������n(H2)��n(CO)�Ĺ�ϵ��ͼ��ʾ���¶Ȳ��䣬��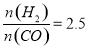 ʱ���ﵽƽ��״̬��CH3OH���������������ͼ���е�______�㡣
ʱ���ﵽƽ��״̬��CH3OH���������������ͼ���е�______�㡣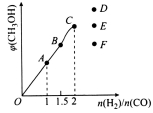
��5��Ϊ�����ȼ�ϵ������ʿ��Խ��״����Ϊȼ�ϵ�أ�д��KOH���������Һʱ���״�ȼ�ϵ�صĸ�����Ӧʽ��___________________���õ�ظ�����ˮ�����բ����ʱ�����Ա�����բ������ʴ�����ֵ绯ѧ������������___________��
��6�����м״��ķ�ˮ�����ŷŻ����ˮ��Ⱦ������ClO2��������ΪCO2��Ȼ���ټӼ��кͼ��ɡ�д�������״����Է�ˮ�����У�ClO2��״���Ӧ�����ӷ���ʽ��___________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com