
���������Լ�����̪��ʯ�ϡ���ᡢ
CH3COONa���塢Ũ���ᡢCa(OH)2��ĩ��CH3COONH4���������ˮ�����ݣ���������ˮ�д��ڵ���ƽ�⣻������������ӻ��ƻ�ˮ�ĵ���ƽ�⣮������ƽ����ƶ���������ָʾ����ɫ�ı仯���ɴ˿ɷֱ�ѡ�������Լ����������ʵ�鷽��֤��CH3COOH�����ᣬ�����ʵ�鷽��������(1)Ӧ�ø��ݢٵ�ʵ�鷽��������________��
(2)Ӧ�ø��ݢڵ�ʵ�鷽��������________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ʡ�����и�����ѧ����ĩ����������ѧ�Ծ��������棩 ���ͣ������
��11�֣�ij��ɫ��Һ�к���K����Cl����OH���� ��
�� ��Ϊ����ȷ�����������ĸ��������ӣ����õ��Լ��У�ϡ���ᡢϡ���ᡢ��������Һ�����ᱵ��Һ������ʯ��ˮ�ͷ�̪��Һ�����м���OH����ʵ�鷽�����ԣ���֪�������������ӵĹ�������ͼ��ʾ��
��Ϊ����ȷ�����������ĸ��������ӣ����õ��Լ��У�ϡ���ᡢϡ���ᡢ��������Һ�����ᱵ��Һ������ʯ��ˮ�ͷ�̪��Һ�����м���OH����ʵ�鷽�����ԣ���֪�������������ӵĹ�������ͼ��ʾ��
�������ʵ��������������������и��⣺
��1��ͼ������a��b��c����������������ӷֱ��ǣ�
�� a_________________, b___________________��c_____________________��
��2����ɫ����A�ӹ����Լ��ڷ�����Ӧ�����ӷ���ʽ�ǣ�___________________��
��3����ɫ��ҺA���Լ��۵���ҪĿ���ǣ�_____________________________________��
��4����ɫ����A�����Լ��۶������Լ��ڶ�ʵ��������Ӱ�죨ѡ���Ӱ�족������Ӱ��"����ȷ������____________________________��
��5������Bͨ���Լ����з�����Ӧ�����ӷ���ʽ�ǣ�____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ�����и�����ѧ����ĩ����������ѧ�Ծ��������棩 ���ͣ������
��11�֣�ij��ɫ��Һ�к���K����Cl����OH����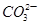 ��
��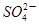 ��Ϊ����ȷ�����������ĸ��������ӣ����õ��Լ��У�ϡ���ᡢϡ���ᡢ��������Һ�����ᱵ��Һ������ʯ��ˮ�ͷ�̪��Һ�����м���OH����ʵ�鷽�����ԣ���֪�������������ӵĹ�������ͼ��ʾ��
��Ϊ����ȷ�����������ĸ��������ӣ����õ��Լ��У�ϡ���ᡢϡ���ᡢ��������Һ�����ᱵ��Һ������ʯ��ˮ�ͷ�̪��Һ�����м���OH����ʵ�鷽�����ԣ���֪�������������ӵĹ�������ͼ��ʾ��
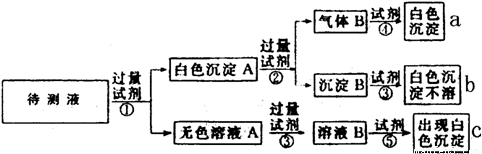
�������ʵ��������������������и��⣺
��1��ͼ������a��b��c����������������ӷֱ��ǣ�
�� a_________________, b___________________��c_____________________��
��2����ɫ����A�ӹ����Լ��ڷ�����Ӧ�����ӷ���ʽ�ǣ�___________________��
��3����ɫ��ҺA���Լ��۵���ҪĿ���ǣ�_____________________________________��
��4����ɫ����A�����Լ��۶������Լ��ڶ�ʵ��������Ӱ�죨ѡ���Ӱ�족������Ӱ��"����ȷ������____________________________��
��5������Bͨ���Լ����з�����Ӧ�����ӷ���ʽ�ǣ�____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��0116 ��ĩ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com