
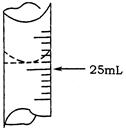
| ʵ����� | ����NaOH��Һ�����/mL | 0.1000mol��L-1HCl��Һ�����/mL | |
| �ζ�ǰ�̶� | �ζ���̶� | ||
| 1 | 25.00 | 0.00 | 26.11 |
| 2 | 25.00 | 1.56 | 31.30 |
| 3 | 25.00 | 0.22 | 26.31 |
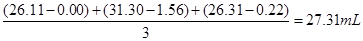
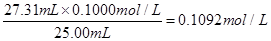


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ޢۢڢݢܢ� | B���٢ޢܢݢڢۢ� |
| C���٢ޢܢڢݢۢ� | D���٢ޢۢܢݢڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����pH��ֽ������ˮʪ����ij��Һ��pH |
| B������500mL 0.10mol/L NaCl��Һ������ʱ���� |
| C���Ա�������Һ�ζ�δ֪Ũ�ȵ�NaOH��Һʱ����ʽ�ζ���δ�ñ���Һ��ϴ |
| D���ñ�NaOH��Һ�ζ�δ֪Ũ�ȵ�������Һ����ʼʱ�ζ��ܼ��촦û�����ݣ�����ʱ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ | B��FeCl3��Һ | C����ˮ | D�����Ȼ�̼ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
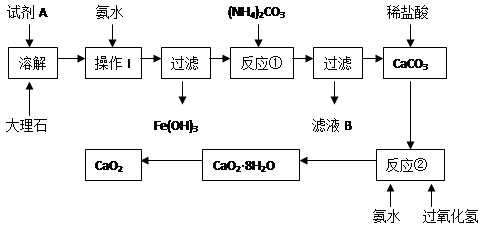
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com