
 ��һƿ������Һ�����п��ܺ���NH4+��K+��Ba2+��Al3+��Fe3+��Mg2+��I-��NO3-��CO32-��SO42-��AlO2-��ȡ����Һ��������ʵ�飺
��һƿ������Һ�����п��ܺ���NH4+��K+��Ba2+��Al3+��Fe3+��Mg2+��I-��NO3-��CO32-��SO42-��AlO2-��ȡ����Һ��������ʵ�飺

 ����ѧУ�ֲ����ܲ�ϵ�д�
����ѧУ�ֲ����ܲ�ϵ�д� �ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
�ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��c��H+����c��OH-��=1��2����Һ��K+��Al3+��HCO3-��ClO- |
| B����Al��Ӧ����H2����Һ��Na+��Ba2+��NO3-��Cl- |
| C��������Һ�У�SO32-��S2-��Fe3+��Cl- |
| D����ʹ��ɫ��̪����ɫ����Һ��K+��Ca2+��Cl-��HCO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ˮ�������ƶ�����Ư�����ã���Ư��ԭ������ |
| B����ѧ��Ӧ 2Na2O2+2H2O=4NaOH+O2�����������ֻ�����Ӧ���͵�����������ԭ��Ӧ |
| C���������Ķ���������20mL12 mol?L-1�������ϼ��ȣ���ַ�Ӧ���������������ʵ���Ϊ0.06mol |
| D���⻯���������˹����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��NH3?H2O�TNH4++OH- |
| B��KClO3�TK++Cl-+3O2- |
| C��CH3COOH�TCH3COO-+H+ |
| D��NaOH�TNa++OH- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ʯ��ˮ�����̼��������Һ��Ӧ��HCO3-+Ca2++OH-�TCaCO3��+H2O |
| B������������Һ�м�������������Һ�����ԣ�2H++SO42-+Ba2++2OH-�TBaSO4��+2H2O |
| C������ͭ��ϡ���ᷴӦ��2H++O2-�TH2O |
| D��̼��������Һ�м������CO32-+2H+�TCO2��+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������������������ά |
| B���轺����������� |
| C�����������ζƷ |
| D��Ũ�������������̲����ƹ���Ʒ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��CaO+H2O=Ca��OH��2 | ||||
B��C+H2O��g��
| ||||
| C��2F2+2H2O=4HF+O2 | ||||
| D��3NO2+H2O=2HNO3+NO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
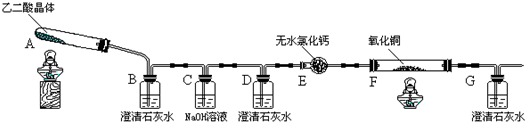
| �ζ�ǰ | ��һ���յ� | �ڶ����յ� | �������յ� | ���Ĵ��յ� | |
| �ζ��� Һ��̶� | 0.00mL | 20.02mL | 21.00mL | 19.98mL | 20.00mL |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com