
�����±�����������һ���Ҵ�ȼ�������������Ѿ�ͻ��200������Ϊ�˸��õ��ƹ��Ҵ�ȼ��������ʹ�ã�����������������ͨ��������������16%������˰�������Ҵ�ȼ������������ֻ����14%������˰�������ش������ޣ��������ϸ���������Ĺ���֮һ���Ҵ��ɴӸ����������ӹ���������������������ͼ��ʾ��
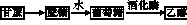
(1)д������ת��Ϊ�����ǵĻ�ѧ����ʽ����������������������������������������
(2)�Ҵ���������ȼ���⣬�������������ϳ������л����ͼ�����Ҵ�Ϊ��ʼԭ�ϵ�ת����ϵͼ������A�����ϩ������ش��������⣺
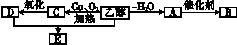
��B�Ǹ߷��ӻ�������ճ������г�����ʳƷ��װ����������������������⣬д����A�ϳ�B�Ļ�ѧ����ʽ��������������������������������
��E��һ�־�����ζ��Һ�壬�����ʽΪC4H8O2��д���Ҵ���D��Ӧ����E�Ļ�ѧ����ʽ��������������������������������������������������
��д���Ҵ�����C�Ļ�ѧ����ʽ��������������������������������������
 Сѧ��10���ӿ������100��ϵ�д�
Сѧ��10���ӿ������100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��״���£��ܱ������ڷֱ���������H2��O2�Ļ�������ڿ��ƶ��Ļ������ߣ�����ʱ�����ͼ��ʾ������װ���ұ�H2��O2�Ļ�������ȼ���������������ָ���ԭ�¶Ⱥ����һ�����ͣ�������������룬��ԭ��H2��O2���������ӽ���(����)
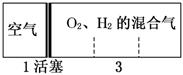
��2��7���� ��5��4���� ��4��5���� ��7��2
A���٢� B���ڢ�
C���ۢ� D���٢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӷ���ʽ��д������ǣ� ��
A.��ϡ�������ͭƬ�ϣ�Cu+2H+��Cu2++H2��
B.��NaOH��Һ�е��뼸��NH4Cl��NH4++OH-��NH3��+H2O
C.������ͭм����Ũ�����У�Cu+2NO3-+4H+��Cu2++2NO2��+2H2O
D.����ͨ��������: NH3+ H+��NH4+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����, ��ȷ���ǣ�������
A.Һ̬��ֲ���;����������������Ӳ����
B.��Ȼ��֬������ɵ��������������
C.��֬������������ˮ��, ����߷����IJ���
D.֬����������������Ժ�����, �۵��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ӷ��²˵�ը���ᡢ�����ܲ赽��ͯ�̷�, ����ʳƷ�����ı����в����Т١��°�ũҩ���ڡ��յ��족���ۡ���Ԫ�ء����ܡ��������Ρ��Ȼ�ѧ���ʳ���. ҵ����ʿ������, ��ѧ����Ⱦ����ΪΣ��ʳƷ��ȫ��һ��ɱ�֡�. ������ѧ�������ᵽ��������, ��ijʳƷ���Ӽ����������е���������ʱ���������ж����ǣ�������
A.�ڢۢ� B.�ڢ� C.�ۢ� D.ֻ�Т�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C�Ǵ����Ϥ�������˶�������ص����ֻ������������Ԫ�ز��������֣���������ת����ϵ��
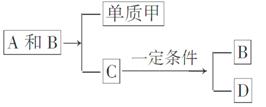
���л�����DҲ���ճ������г����Ļ������һ�������¿��뵥�ʼ�һ���������±仯��
D��3�רD��3A��2B
�ش��������⣺
(1)��A��B��C��D���ֻ������У�����Ԫ����ͬ����(д��������)________��________��
(2)�����£�A��B��ͨ��ʲô;��ת��ΪC�ģ�
____________________________________________________��
(3)Ŀǰ��������B����Ȼ���еĺ������������ƣ��Ի��������˲�����Ӱ�죬�������ֱ仯����Ҫԭ����__________________
___________________________________________________________________________________________________________________��
(4)������D����һ��ͬ���칹�壬����ͬ���칹��Ľṹ��ʽ��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NAΪ�����ӵ�������ֵ������������ȷ����
A��7.8 g��������������������̼��Ӧת�Ƶĵ�����Ϊ0.1NA
B��1 mol��L��1��̼������Һ�к���CO32������ĿС��NA
C����״���£�2.24LCl2����ˮ��ת�Ƶĵ�����ĿΪ0.1NA
D��1molNa����ȫ��������Na2O2��ʧȥ��2 NA����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ܴﵽʵ��Ŀ���ǣ�������
| �� | A�� |
����HCl������ | B�� |
��ȡ��������ˮ |
| �� | C�� |
CaCO3��ϡ������ȡCO2 | D�� |
��ȥ��������CO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ij�ܱ������м���0.3 mol A��0.1 mol C��һ������B�������塣һ�������·�����Ӧ�������ʵ�Ũ����ʱ��仯���ͼ��ʾ[t0��t1�ε�c(B)�仯δ����]����ͼΪt2ʱ�̺�ı�����ƽ����ϵ�������淴Ӧ������ʱ��仯����������ĸ��ζ����ı�һ�ַ�Ӧ�����һ�����ͬ��t3ʱ��Ϊʹ�ô���������˵������ȷ����
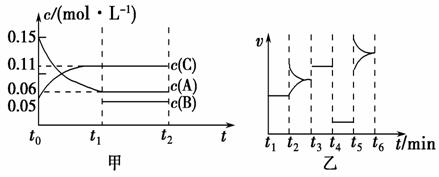
A����t1��15 s����A��Ũ�ȱ仯��ʾt0��t1�ε�ƽ����Ӧ����Ϊ0.004 mol��L��1��s��1
B�� t4��t5�θı������һ��Ϊ��Сѹǿ
C�����������ݻ�Ϊ2 L��B����ʼ���ʵ���Ϊ0.02 mol
D��t5��t6�Σ�������A�����ʵ���������0.06 mol�����˹����������������Ƚ�������Ϊa kJ���÷�Ӧ���Ȼ�ѧ����ʽ3A(g) B(g)��2C(g)��H����50a kJ��mol��1
B(g)��2C(g)��H����50a kJ��mol��1
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com