
A��B��C�Ǵ����Ϥ�������˶�������ص����ֻ������������Ԫ�ز��������֣���������ת����ϵ��
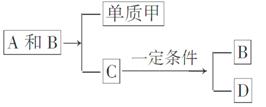
���л�����DҲ���ճ������г����Ļ������һ�������¿��뵥�ʼ�һ���������±仯��
D��3�רD��3A��2B
�ش��������⣺
(1)��A��B��C��D���ֻ������У�����Ԫ����ͬ����(д��������)________��________��
(2)�����£�A��B��ͨ��ʲô;��ת��ΪC�ģ�
____________________________________________________��
(3)Ŀǰ��������B����Ȼ���еĺ������������ƣ��Ի��������˲�����Ӱ�죬�������ֱ仯����Ҫԭ����__________________
___________________________________________________________________________________________________________________��
(4)������D����һ��ͬ���칹�壬����ͬ���칹��Ľṹ��ʽ��________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ������Ҫ0.1 mol/L NaOH��Һ450 mL��0.5 mol/L������Һ500 mL��������������Һ����������ش��������⣺
����ͼ��ʾ�������У�������Һ�϶�����Ҫ����__________________________ (�����)������������Һ�����õ��IJ��������� (����������)��
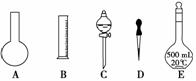
������0.1 mol/L NaOH��Һʱ������ȷ�IJ���˳���ǣ���ĸ��ʾ��ÿ����ĸֻ����һ�Σ� ��
A��������ƿ�ǽ�����ҡ��
B�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�������
C������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1~2cm��
D����30mLˮϴ���ձ�2~3�Σ�ϴ��Һ��ע������ƿ
E�����ܽ������������Һ�ز�����ע��500mL������ƿ��
F��ȷ��ȡ���������������ƹ������ձ��У��ټ�������ˮ��Լ30mL�����ò���������������ʹ�����ܽ�
������0.1 mol/L NaOH��Һʱ����ʵ����������������ȷ��������ʱ��������ƿ�̶��ߣ���������ҺŨ�� 0.1 mol/L(����ڡ��������ڡ���С�ڡ�)��
������0.5 mol/L������Һ500 mLʱ��������������Ϊ98%���ܶ�Ϊ1.84 g/cm3��Ũ��������Ϊ mL(����������һλС��)��
������0.5 mol/L������Һʱ����ʵ����������������ȷ��������Ͳ��ȡŨ����ʱ���ӿ̶��ߣ���������ҺŨ�� 0.5 mol/L(����ڡ��������ڡ���С�ڡ�)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
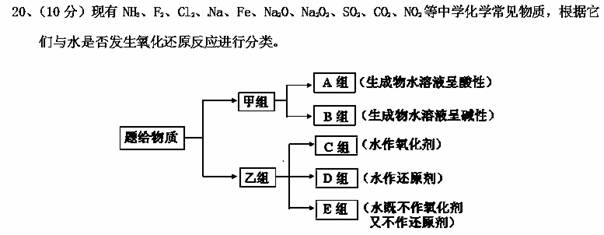
��ش��������⣺
��1��ͼ����ʾ�ķ������_________________________��
��2������ɫ����λ��_____�飨�A~E������1mol��������ˮ��Ӧת�Ƶ���_______mol��
��������������ѧ��������Ϊ_____������ĸ����A���Ӽ�B���Լ�C�Ǽ��Լ���
��3���õ���ʽ��ʾA�����ܽ�Ƚ�С���ʵ��γɹ��̣�
___________________________________________________________________________��
��4��C����ijһ�������ڼ���ʱ������ˮ������Ӧ���仯ѧ����ʽΪ��
___________________________________________________________________________��
��5��д��D��������һ��������ˮ��Ӧ�����ӷ���ʽ�� _________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѻƬ��������� �� ��
A.ѻƬζ��
B.����ѹ�ȵ���ڻ�����
C.����ij������Ĵ̼�
D.ѻƬʹ��ʳ��ǿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����±�����������һ���Ҵ�ȼ�������������Ѿ�ͻ��200������Ϊ�˸��õ��ƹ��Ҵ�ȼ��������ʹ�ã�����������������ͨ��������������16%������˰�������Ҵ�ȼ������������ֻ����14%������˰�������ش������ޣ��������ϸ���������Ĺ���֮һ���Ҵ��ɴӸ����������ӹ���������������������ͼ��ʾ��
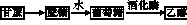
(1)д������ת��Ϊ�����ǵĻ�ѧ����ʽ����������������������������������������
(2)�Ҵ���������ȼ���⣬�������������ϳ������л����ͼ�����Ҵ�Ϊ��ʼԭ�ϵ�ת����ϵͼ������A�����ϩ������ش��������⣺
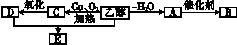
��B�Ǹ߷��ӻ�������ճ������г�����ʳƷ��װ����������������������⣬д����A�ϳ�B�Ļ�ѧ����ʽ��������������������������������
��E��һ�־�����ζ��Һ�壬�����ʽΪC4H8O2��д���Ҵ���D��Ӧ����E�Ļ�ѧ����ʽ��������������������������������������������������
��д���Ҵ�����C�Ļ�ѧ����ʽ��������������������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���л�ѧʵ����ʵ���������ȷ����
A�����ˮ�еμ�CCl4�����ã�CCl4����Ϻ�ɫ��˵������CCl4�ӵ�ˮ����ȡ��
B��Cl2��SO2����ʹƷ����Һ��ɫ��˵�����߾���������
C���ò�˿պȡ����ij��Һ������ɫ��Ӧ������ʻ�ɫ������Һһ����������Һ
D�������£��ý��������Ȼ��ؿ����û��������أ�˵���ƵĽ����Աȼ�ǿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ú������Ʊ����ʵ��ʵ���У��漰�IJ�����ȷ���ܴﵽʵ��Ŀ�ĵ���
|
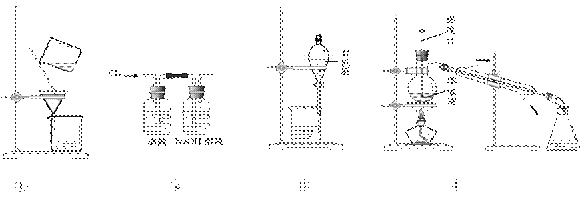
A����ͼ����ʾװ�ã����˺����ҽ�ȡҺ
B����ͼ����ʾװ�ã�����Һ��ͨ��Cl2
C����ͼ����ʾװ�ã��ȷų��²�Һ�壬�ٷų��л���
D����ͼ����ʾװ�ã�������ȡ��������õ��ʵ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NA��ʾ�����ӵ�������������������ȷ���ǣ�������
| �� | A�� | ���³�ѹ�£�32 g O2��O3�Ļ����������ԭ����ΪNA |
| �� | B�� | ��״���£�1.8g��H2O�к��еĵ�����ΪNA |
| �� | C�� | ���³�ѹ�£�11.2L����������ԭ����ΪNA |
| �� | D�� | 10����������ԭ����ԼΪ6.02��1023 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͼ��ʾ���ס���֮��ĸ���K�ͻ���F���������ƶ������г���2molA��1molB�����г���2molC��1molHe����ʱKͣ��0������һ�������·������淴Ӧ��
2A��g��+B��g�� 2C��g���� ��Ӧ�ﵽƽ����ٻָ���ԭ�¶ȡ��ش��������⣺
2C��g���� ��Ӧ�ﵽƽ����ٻָ���ԭ�¶ȡ��ش��������⣺
��1���ﵽƽ��ʱ������K����ͣ����0�̶����a������a��ȡֵ��Χ�� ��
��2�����ﵽƽ��ʱ������K����ͣ�������̶�1������ʱ���ݻ�Ϊ2L����Ӧ��ѧƽ�ⳣ��Ϊ___________,
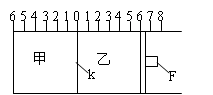 ��3�����ﵽƽ��ʱ������K����ͣ�������̶ȿ���0���� �����п��ƶ�����F����ͣ�����Ҳ�Ŀ̶Ȳ����� ��
��3�����ﵽƽ��ʱ������K����ͣ�������̶ȿ���0���� �����п��ƶ�����F����ͣ�����Ҳ�Ŀ̶Ȳ����� ��
����һ��ʼ�ͽ�K��F�̶����������������䣬��ﵽ
ƽ��ʱ��
��1����ü���A��ת����Ϊb��������C��ת����Ϊ ��
��2�������ҡ����������е�ѹǿ����d��ʾ����d��ȡֵ��Χ�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com