
������BN����һ����Ҫ�Ĺ����մɲ��ϡ�����Ȼ��ɰΪ��ʼ�����һϵ�з�Ӧ���Եõ�BF3��BN����ش���������
��1����̬Bԭ�ӵĵ����Ų�ʽΪ_________��B��N��ȣ��縺�Խϴ����_________��BN��BԪ�صĻ��ϼ�Ϊ_________��
��2����BF3�����У�F-B-F�ļ�����_______��Bԭ�ӵ��ӻ��������Ϊ_______��BF3����NaF���ÿ�����NaBF4��BF4��������ṹΪ_______��
��3������ʯī�ṹ���Ƶ��������������У�����Bԭ����Nԭ��֮��Ļ�ѧ��Ϊ________�����������Ϊ________��
��4�������������ڸ��¸�ѹ�£�����ת��Ϊ������������ṹ����ʯ���ƣ�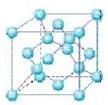 Ӳ������ʯ�൱�������߳�Ϊ361.5pm�������������к���______����ԭ�ӡ�________����ԭ�ӣ�������������ܶ���_______g��cm-3��ֻҪ������ʽ�����ؼ������ֵ������٤������ΪNA����
Ӳ������ʯ�൱�������߳�Ϊ361.5pm�������������к���______����ԭ�ӡ�________����ԭ�ӣ�������������ܶ���_______g��cm-3��ֻҪ������ʽ�����ؼ������ֵ������٤������ΪNA����
��(1)1s22s22p1�� N�� +3 (2)120�� �� sp2�� ��������
(3)���ۼ�(���Թ��ۼ�)�� ���Ӽ���
(4)4�� 4�� 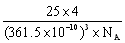
���������������1��5��Ԫ��B�Ļ�̬Bԭ�ӵĵ����Ų�ʽΪ1s22s22p1��B��N��ȣ�����ԭ�Ӱ뾶B>N���ǽ�����N>B�����Ե縺�Խϴ����N����BN��BԪ�صĻ��ϼ�Ϊ+3�ۡ���2��BF3������ƽ���������η��ӡ���BF3�����У�F-B-F�ļ�����120�㡣Bԭ�ӵ��ӻ��������Ϊsp2��BF3����NaF���ÿ�����NaBF4����BF4����Bԭ�ӵ��ӻ���ʽΪsp3����������ṹΪ�������塣��3������������ʯī�ṹ���ơ��ڲ���Bԭ����Nԭ��֮��Ļ�ѧ��Ϊ���Թ��ۼ����ڲ���������Ƿ��Ӽ�����������4������������Ľṹ����ʯ���ƣ�Ӳ������ʯ�൱���ڽ��ʯ��һ�������к���C��8��1/8��6��1/2+4=8.���������������к���4����ԭ�ӡ�4��ԭ�ӣ����ھ����߳�Ϊ361.5pm������������������ܶ���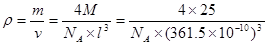 g��cm-3��
g��cm-3��
���㣺����ԭ�ӡ����Ӽ�����Ľṹ�������ܶȵļ����֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ԫ��B�ڻ�ѧ���к���Ҫ�ĵ�λ����Ļ�������ũҵ��ҽԺ�������ȷ�����;�ܹ㡣��ش��������⣺
��1��д����BԪ��ͬ�����GaԪ�صĻ�̬ԭ�Ӻ�����ӷֲ�ʽ ����ԭ�ӽṹ�ĽǶȷ�����B��N��OԪ�صĵ�һ�������ɴ�С��˳��Ϊ ��
��2������������������˹������ڸ��¸�ѹ�����ºϳɣ����ڳ�Ӳ���ϣ�ͬ��ԭ�Ӿ���ĵ�����BN���Ⱦ������и���Ӳ�Ⱥ������Ե�ԭ���� ��
��3����BF3����������ԭ�ӵ��ӻ���������� ��SiF4���Ŀռ乹���� ��
��4����ѧ�ҷ�����þ��39Kʱ�ʳ����ԣ�����þ���������ģ���У�þԭ�Ӻ���ԭ���Ƿֲ��Ų��ģ�һ��þһ����������С�ͼ23�Ǹþ��������ȡ���IJ���ԭ����Z�᷽���ͶӰ��������þԭ��ͶӰ����������ԭ��ͶӰ��ͼ�е���ԭ�Ӻ�þԭ��ͶӰ��ͬһƽ���ϡ�����ͼʾȷ����þ�Ļ�ѧʽΪ ��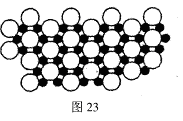
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��14�֣�Ԫ�����ڱ���ѧϰ��ѧ����Ҫ���ߣ���������������Ϣ���ɣ������ǰ��ֶ�����Ԫ�ص������Ϣ����֪���ԭ�Ӱ뾶Ϊ0.089nm��
| Ԫ�ش��� | A | B | C | D | E |
| ԭ�Ӱ뾶/nm | 0.16 | 0.143 | 0.102 | 0.099 | 0.074 |
| ��Ҫ���ϼ� | +2 | +3 | +6��-2 | -1 | -2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��15�֣�
������Ԫ��X��Y��Z��W�����ڱ��е�λ����ͼ��ʾ������W���������������γɵ���Ҫ���ʡ�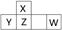
��1�� д��W��ԭ�ӽṹʾ��ͼ�� ��
��2�� ������X�����������ͨ�뺬YԪ�ص���������Һ�С���Ӧ�����ӷ���ʽΪ ��
��3�� ��֪:��X(s) + O2(g) ��XO2(g)�� ��H����393.5 kJ��mol��1
��H2(g) + 1/2 O2(g) ��H2O(g)���� ��H����242.0 kJ��mol��1
��XH4(g) + 2O2(g) ��XO2(g) + 2H2O(g) ��H����802.0 kJ��mol��1
��XH4����ֽ��������X������Ȼ�ѧ����ʽΪ�� ������
��4�� ZO���ɵ���X��ZO2��Ӧ��ȡ����Z���м�����������ʱ��ZO��NaOH��Һ��Ӧ(���ﺬ��һ�ֹ��嵥�ʺ�һ������)�Ļ�ѧ����ʽΪ_______________��
��5�� ����ԭ���ԭ��������W��һ�������O2��H2O���Ʊ�W������������Ӧˮ���д���õ�ظ�����Ӧʽ��___________��
��6�� ��W����̬�⻯��ͨ��һ������NaOH��Һ�У���������Һ����μ���ϡ����������������������HCl�����ʵ����Ĺ�ϵ��ͼ��ʾ(����������ܽ��HCl�Ļӷ�)��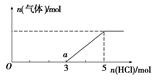
��O����Һ���������ʵĻ�ѧʽΪ____________��
��a����Һ�У�c(Na+): c(Cl��)= _______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��I���¹���������ѧ�������Ƴ���20��̼ԭ����ɵĿ�����״����C20������״�ṹ��������������ι�����ͼ��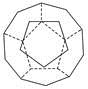
��C20������ÿ��̼ԭ��ֻ�����ڵ�3��̼ԭ���γɻ�ѧ����
�ڶ�����Ķ�������������������Ĺ�ϵ����ѭŷ�����������������������������2,
��ش� C20���ӹ���________��������Σ�����________����ߡ�
II.A��B��C�Ƕ����ڷǽ���Ԫ�أ��˵������������Aԭ����Χ�����Ų�Ϊns2np2��C�ǵؿ��к�������Ԫ�ء�DԪ�صĺ˵����Ϊ29�����ö�Ӧ��Ԫ�ط��Ż�ѧʽ��գ�
(1)A��B��C�ĵ�һ��������С�����˳��Ϊ________��
(2)����(AB)2�м����֮��ļн�Ϊ180�㣬���жԳ��ԣ�ÿ��ԭ������������������˵��ӣ���ṹʽΪ________��1 mol�÷����к��Цм�����ĿΪ________���÷�����̼ԭ�ӵ��ӻ����������__________________���÷�������________����(����ԡ��Ǽ��ԡ�)��
(3)��̬Dԭ�ӵĵ����Ų�ʽΪ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��12�֣�A��B��C��D��E��F����Ԫ�ط������������ڣ���ԭ��������������A��Dͬ���壬���γ����ӻ�����X��B���⻯����F���⻯��ɷ�Ӧ�������ӻ�����Y�� ��B�ĵ����ǿ����к�����ߵ����ʣ�Cԭ�ӵ����������Ǵ�����������3����D��E��F 3��ԭ������㹲��11�����ӣ� ����3��Ԫ�ص�����������ˮ�����������ܷ�����Ӧ�����κ�ˮ��
��1��BԪ�ص�������________��B���ʵĽṹʽΪ_____________��
��2���õ���ʽ��ʾ������X���γɹ���__________________________________��
��3��������Y�ĵ���ʽΪ_____________��A2C2�ĵ���ʽΪ_____________��
��4��D��E����������ˮ����֮�䷴Ӧ�����ӷ���ʽΪ ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��֪A��B��C��D��E��FΪԪ�����ڱ���ԭ���������������ǰ20��Ԫ�أ�A��B��C��D��E��F�ֱ�λ��ͬһ���ڡ�Aԭ��L������3��δ�ɶԵ��ӣ�B�ļ�����������Cԭ�Ӻ�������Ų���ͬ������DԪ�ص��ε���ɫ��Ӧ����Ϊ��ɫ��EF3���ɻ��ý����ͻ��÷ǽ�����ɵĹ��ۻ����G�ж������������һ���������д��ԣ���д���пհ�
(1)Ԫ�صĵ�һ������������________�����ڹ���Ԫ�ص���________(��дԪ�ط���)
(2)д��BԪ�صĻ�̬ԭ�Ӽ۵����Ų�ʽ__________________��F���ӵ����Ų�ʽ_________________��
(3)AF3������Aԭ�ӵ��ӻ�������________��AF3���ӵļ��ι���Ϊ___________��
(4)��֪E2B3�ľ����DF�ľ����ܴ�ö࣬�Է����������߾����ܲ������Ҫԭ���ǣ�________________________________________________________��
(5)����G���������________��G�ľ����ṹ����ͼ����ʾ��G�ľ���Ϊ________�ṹ����Gԭ�ӵİ뾶Ϊ1.27��10��10 m��G���������еľ������ȣ�����ͼ����AB�ij���Ϊ________m��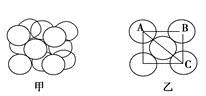
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijͬѧ��̽�����̼ԭ�ӵĵõ���������ǿ����ͨ���Ƚ���������������Ӧˮ���������ǿ������֤���������ͼʵ�飬��ش�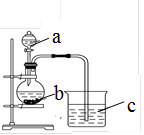
��1������a�������� ��Ӧʢ������ҩƷ�е� ������ţ���
A��ϡ���� B. ������ C. ������ D. ����
��2������bӦʢ������ҩƷ�е� ������ţ���
A��̼��� B. ������ C. �Ȼ��� D. ̼����
��3��b�з�Ӧ�����ӷ���ʽΪ ��
����������C�е������� ������֤�� �� ����ǿ���ѧʽ�����õ��ӵ����� �� ǿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���и��黥Ϊ�ȵ��������
| A��N2O��NO2 | B��O3��SO2 | C��CH4��NH3 | D��OH-��NH2- |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com