
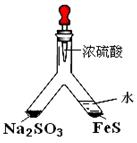
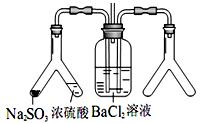
 ��Ŀ�ģ�̽�� SO2��BaCl2��Ӧ������������������������ͨ���Ȼ�����Һ����������������ͨ����һ���������Բ�����ɫ���������Ҳ� Y����Ӧ���õ�ҩƷ��____________��___________����Ҫʱ���Լ��ȣ����ó����Ļ�ѧʽΪ________________��
��Ŀ�ģ�̽�� SO2��BaCl2��Ӧ������������������������ͨ���Ȼ�����Һ����������������ͨ����һ���������Բ�����ɫ���������Ҳ� Y����Ӧ���õ�ҩƷ��____________��___________����Ҫʱ���Լ��ȣ����ó����Ļ�ѧʽΪ________________��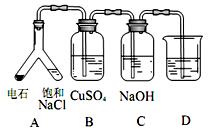
 ֤��ʯ������Ҫ�ɷ֣�D��ʢ��______________��
֤��ʯ������Ҫ�ɷ֣�D��ʢ��______________��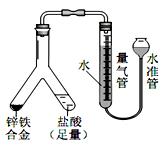


 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ����ش��������⣺
����ش��������⣺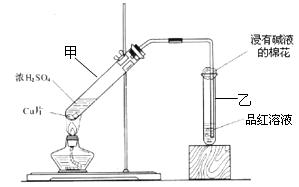
 L��Ba(OH)2��Һ�����ٲ�������ʱ��ǡ������40.00 mL Ba(OH)2��Һ������㣺
L��Ba(OH)2��Һ�����ٲ�������ʱ��ǡ������40.00 mL Ba(OH)2��Һ������㣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
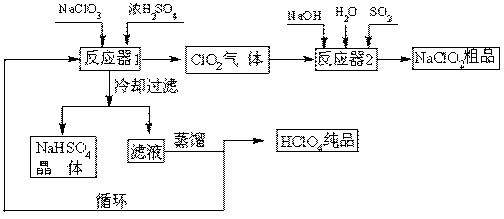
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
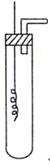
 �����ƶ������Թ�Һ���У�ʹ֮�����ᷴӦ����Ӧ�����ӷ���ʽ�� ��
�����ƶ������Թ�Һ���У�ʹ֮�����ᷴӦ����Ӧ�����ӷ���ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| �ζ����� | ������Һ�����/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00 | 1.02 | 21.03 |
| 2 | 25.00 | 2.00 | 21.99 |
| 3 | 25.00 | 0.20 | 20.20 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ô����ƾ���ͼ�ʯ�ҹ����Ƽ��� |
| B������м����ˮ����������屽 |
| C���ڱ�����Һ�е�������ϡ��ˮ���ְ�ɫ���� |
| D����ͭ˿�ھƾ����ϼ��Ⱥ�����������ˮ�Ҵ��У�ͭ˿�ָ���ԭ���ĺ�ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
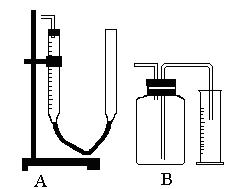
| A����B����ǰ�����ƿ��ͨ��Ӧʢ����Ӧ��Һ�� |
| B����A����ǰ����������Ӧ������Ӧ��Һ�� |
| C���ռ���������ڶ���ǰӦʹʢҺ���ڣ���Ͳ��Һ���뼯���ܣ�ƿ�����������Һ����ƽ |
| D��A��ֱ�Ӳ������������Bֱ�Ӳ�������Һ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
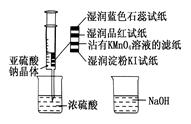
| A����ɫʯ����ֽ�ȱ�����ɫ |
| B��Ʒ����ֽ��մ��KMnO4��Һ����ֽ����ɫ֤����SO2����Ư���� |
| C��ʪ�����KI��ֽ����˵��SO2������������I2 |
| D��NaOH��Һ�����ڳ�ȥʵ���ж����SO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������BaCl2��Һ�а�ɫ�����������ټ����ᣬ��������ʧ��ԭ��Һһ����SO42�� |
| B������KMnO4������Һ���Ϻ�ɫ��ȥ��֤��CH2=CHCH2CHO��һ����̼̼�����ͼ� |
| C��ij��ɫ������ʹʪ��ĺ�ɫʯ����ֽ�������������ˮ��Һһ���Լ��� |
| D������ϡ���ᣬ������ɫ����ʹ����ʯ��ˮ����ǵ����壬ԭ��Һһ����CO32����SO32�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com