
 ��2011?ɽ�������С������г��漰�ơ����仯���
��2011?ɽ�������С������г��漰�ơ����仯���


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
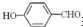 ��
�е��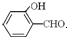 �ߣ�ԭ����
�ߣ�ԭ����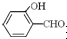 �γɷ������������
�γɷ������������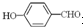 �γɷ��Ӽ���������Ӽ����ʹ���Ӽ�����������
�γɷ��Ӽ���������Ӽ����ʹ���Ӽ����������� �γɷ������������
�γɷ������������ �γɷ��Ӽ���������Ӽ����ʹ���Ӽ�����������
�γɷ��Ӽ���������Ӽ����ʹ���Ӽ�����������| 224 |
| aNA |
| 224 |
| aNA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��1����2011?���ո߿�����ѡ��Ag2O2����п���Ե�ص������������ʣ���������ҺΪ KOH ��Һ����طŵ�ʱ������Ag2O2ת��ΪAg��������Znת��ΪK2Zn��OH��4��д���õ�ط�Ӧ����ʽ��
��1����2011?���ո߿�����ѡ��Ag2O2����п���Ե�ص������������ʣ���������ҺΪ KOH ��Һ����طŵ�ʱ������Ag2O2ת��ΪAg��������Znת��ΪK2Zn��OH��4��д���õ�ط�Ӧ����ʽ��| 3 |
| 2 |
| 3 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
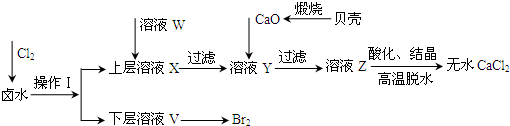
| ��ʼ����ʱ��pH | ������ȫʱ��pH | |
| Mg2+ | 9.6 | 11.0 |
| Ca2+ | 12.2 | c��OH-��=1.8mol?L-1 |
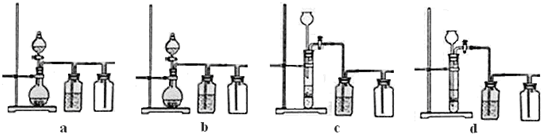
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com