
 ijѧ����0.1000mol/L�������Һ�ⶨij�ռ���Ʒ�Ĵ��ȣ����ʲ������ᷴӦ����ʵ�鲽�����£�
ijѧ����0.1000mol/L�������Һ�ⶨij�ռ���Ʒ�Ĵ��ȣ����ʲ������ᷴӦ����ʵ�鲽�����£�| �ζ����� | ����Һ��� | �����������mL�� | |
| �ζ�ǰ������mL�� | �ζ��������mL�� | ||
| ��һ�� | 20.00 | 0.20 | 20.38 |
| �ڶ��� | 20.00 | 4.00 | 24.20 |
| ������ | 20.00 | 2.38 | a |
���� ��1����������һ�����ʵ���Ũ�ȵ���Һ�����Ʋ���ѡ��ʹ�õ�������
��2����ʢ��Һ����ʽ�ζ��ܱ�����ϴ��������ȡ����ҺŨ��ƫ�ͣ�
�ڸ��ݼ�ʽ�ζ����������ݵķ�����
�۵ζ�ʱ�۾�ע����ƿ����ɫ�仯�����ݴ���Һ�м����̪����ҺΪ��ɫ���кͷ�Ӧ��������Һ��ɫ��ʧ�����жϣ�
�ܵζ�ǰ���ӣ����¶���ƫ�ζ����ӻᵼ�¶���ƫС��
��3�����ݵζ��ܵĽṹ�;�ȷ�ȣ��ȷ������ݵ���Ч�ԣ����ݵζ��ܶ�������������Һ���������������������ƽ�������Ȼ����ݹ�ϵʽNaOH��HCl����������Ƶ����ʵ������ټ����ռ���Ʒ�Ĵ��ȣ�
��� �⣺��1����2.50g�����������ʵĹ����ռ���Ʒ����500mL��Һ�����ƹ���Ϊ�����㡢�������ܽ⡢��ȴ��ת�ơ�ϴ�ӡ�ҡ�ȵȣ���Ҫ��������������ƽ���ձ�����������500mL����ƿ����ͷ�ιܣ����õIJ������������ձ�����ͷ�ιܡ��������⣬����Ҫ500mL����ƿ��
�ʴ�Ϊ��500mL����ƿ��
��2����������ˮϴ����ʽ�ζ��ܣ�Ȼ��������ʽ�ζ����ñ�Һ������ϴ������ᵼ�±�ҺŨ�ȼ�С�����ĵı�Һ���ƫ�ⶨ���ƫ��
�ʴ�Ϊ����ƫ��
�ڼ�ʽ�ζ����������ݵķ������ѵζ��ܵĽ�ͷ�����������������������ݣ�
��ѡ������
�۵ζ�ʱ˫��Ӧע��۲���ƿ����Һ��ɫ�ı仯����ƿ�е����̪����ҺΪ��ɫ�����ŷ�Ӧ���У�����������������ǡ�÷�Ӧ����Һ����ɫ����ʧ�����Դﵽ�յ�����Ϊ�����μ����һ�α�Һʱ������Һ�ɺ�ɫ�պñ�Ϊ��ɫ���Ұ�����ڲ���ɫ��
�ʴ�Ϊ���۲���ƿ����Һ��ɫ�ı仯�����μ����һ�α�Һʱ������Һ�ɺ�ɫ�պñ�Ϊ��ɫ���Ұ�����ڲ���ɫ��
�ܲ���II�����Ӷ������ᵼ�µζ��ܵĶ���ƫ������и��Ӷ������ᵼ�µζ��ܶ���ƫС�����յ������ĵ��������ƫС���ⶨ���ƫ�ͣ�
�ʴ�Ϊ��ƫС��
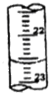
��3���ٵζ�ʱ�ĵζ����е�Һ�棬�����Ϊ22.60mL��
�ʴ�Ϊ��22.60��
������������������ֱ�Ϊ��20.38mL-0.20mL=20.18mL��24.20mL-4.00mL=20.20mL��a=22.6mL��22.6mL-2.38mL=20.22mL�����εζ����ݶ�����Ч�ģ��������������ƽ�����Ϊ��$\frac{20.18ml+20.20ml+20.22ml}{3}$=20.20mL��
���ݹ�ϵʽNaOH��HCl��֪��n��NaOH��=n��HCl��=0.1000mol•L-1��0.022mL=0.00202mol��
����20.00mL������Һ���У�m���ռ�Tn•M�T0.00202mol��40g/mol=0.808g��
����1000mL������Һ����m���ռ�T0.808g��$\frac{500ml}{20ml}$=2.02g��
�ռ�Ĵ��Ȧأ��ռ=$\frac{2.02g}{2.5g}$��100%=80.8%��
�ʴ�Ϊ��80.8��
���� ���⿼������к͵ζ�ʵ�飬��Ŀ�Ѷ��еȣ�ע�����ʵ���ԭ�������衢�����Լ�ע���������ʵ�����������


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
 ij�о�С���ñ�NaOH��Һ�ζ��״ף��ⶨʳ�ð״��д���ĺ���������˵����ȷ���ǣ�������
ij�о�С���ñ�NaOH��Һ�ζ��״ף��ⶨʳ�ð״��д���ĺ���������˵����ȷ���ǣ�������| A�� | ����ͼ����ȡһ������Ĵ���״�����ƿ�� | |
| B�� | �۲��ʽ�ζ��ܶ���ʱ�����ζ�ǰ���ӣ��ζ����ӣ������ᵼ��ʳ�ð״��д���ĺ���ƫС | |
| C�� | ��ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ�������ᵼ��ʳ�ð״��д���ĺ���ƫ�� | |
| D�� | �ζ�ʱ�۾�Ҫע���ŵζ�����NaOH��Һ��Һ��仯����ֹ�ζ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ������ˮ��ʹ����ʯ����ֽ�ȱ�죬����ɫ | |
| B�� | ������ˮ��ֻ����Cl2��H2O���� | |
| C�� | ��ˮ���������������ǿ | |
| D�� | ������ˮ�������ݳ�����������Cl2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢۢܢ� | B�� | �ڢۢݢ� | C�� | ȫ�� | D�� | �٢ڢܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com