����⣺AΪ��ɫ��ĩ����H��C��O��Cu����Ԫ��֤��Ϊͭ�̣������£�DΪ��ɫ��ζ��������G��Ӧ���������ж�DΪCO
2��BΪ��ɫ��ĩ�ƶ�ΪCuO��F��E��Ϊ�л����֪F��E����Է�������֮�����2��˵��EF��Ӧ��������ͭ�������°Ѵ��ǻ�����Ϊȩ����FΪ����EΪȩ����֪A��B��D��E��F��GΪ��ѧ��ѧ�г����Ļ���������ж�EΪCH
3CHO��FΪCH
3CH
2OH��
��1��D��CO
2����G��Na
2O
2����Ӧ�Ļ�ѧ����Ϊ��2Na
2O
2+2CO
2=2Na
2CO
3+O
2���ʴ�Ϊ��2Na
2O
2+2CO
2=2Na
2CO
3+O
2��
��2��B��CuO����F��C
2H
5OH���ڼ��������·�Ӧ����E��CH
3CHO����ȥ��������Ӧ����Ӧ�Ļ�ѧ����ʽΪ��CH
3CH
2OH+CuO
CH
3CHO+H
2O+Cu������F��һ�������ǻ���
�ʴ�Ϊ���ǻ���
��3�����ⶨװ�ü������Լ��������仯��̽��A�Ļ�ѧʽ��
��Ϊʹ����ȷ��Ҫ��ͨ��Ŀ����е�ˮ������ȥ������Ӱ��ⶨ�����װ��Ϊ
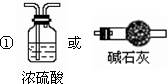
���ʴ�Ϊ��

��
����װ���й��������Ŀ���ǣ���A�ֽ������ˮ��������ʢ��Ũ�����ϴ��ƿ�У���װ����ҩƷ����������ˮ����ͭ�������Ǽ��ˮ�����Ƿ�������ޱ仯֤��ˮ����������A�ֽ������ˮ����ȫ����Ũ�������գ�
�ʴ�Ϊ����A�ֽ������ˮ��������ʢ��Ũ�����ϴ��ƿ�У���ˮ����ͭ��A�ֽ������ˮ����ȫ����Ũ�������գ�
���ж�A����ȫ�ֽ�ķ���Ϊ���������μ��ȡ���������ȴ��������װ�õ����������������0.1 g��֤��A�ֽ���ȫ��
�ʴ�Ϊ���������μ��ȡ���������ȴ��������װ�õ����������������0.1 g��֤��A�ֽ���ȫ��
��ʵ���ó��������ݣ�A���Ⱥ���ȫ�ֽ⣬������8.0g��Ϊ6.0g��װ��������0.9gΪ���ɵ�ˮ�������������������1.1gΪCO
2������ԭ���غ㣬8.0gA�к��е���Ԫ�����ʵ���Ϊ
=0.1mol������̼Ԫ�����ʵ���Ϊ
=0.025mol�����ɵ�����ͭ����Ϊ6g��ͭ���ʵ���Ϊ
=0.075mol����Ԫ�ص�����Ϊ8.0g-0.1mol��1g/mol-0.025mol��12g/mol-0.075mol��64g/mol=2.8g����Ԫ�����ʵ���Ϊ���ʵ���Ϊ=
=0.175mol��A��Ԫ��ԭ�����ʵ���֮��Ϊn��Cu����n��C����n��H����n��O��=0.075��0.025��0.1��0.175=3��1��4��7����ѧʽΪCu
3H
4CO
7����Ϸ�Ӧת����֪���ṹ�к�̼�����ʣ��Ϊ��������д����ѧʽΪ��Cu
3��OH��
4CO
3��CuCO
3?2Cu��OH��
2��
�ʴ�Ϊ��Cu
3��OH��
4CO
3��CuCO
3?2Cu��OH��
2��


 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��

