
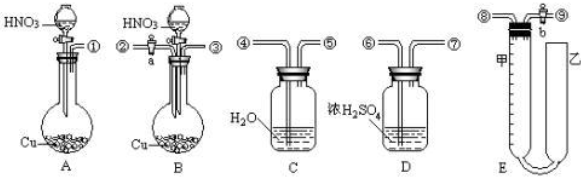
| V |
| 22.4 |
| V-11.2n |
| 33.6n |
| V-11.2n |
| 33.6n |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����Ȼ�����Һ�м�������İ�ˮ��3NH3?H2O+A13+�T3NH4++Al��OH��3�� |
| B��NaOH��Һ��������CO2��OH-+CO2�THCO3- |
| C�����Ȼ�����Һ�м���ͭ�۷����ķ�Ӧ��Cu+Fe3+�TCu2++Fe2+ |
| D��Na2O2��ˮ�ķ�Ӧ��Na2O2+H2O�T2Na++2OH-+O2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��c��H+��=c��OH-��=10-6 mol?L-1��Һ |
| B��pH=7����Һ |
| C��ʹʯ����Һ�ʺ�ɫ����Һ |
| D����ǿ����ǿ������ʵ�����Ӧ�õ�����Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����ʴ��ͱ�����������ȫ��ͬ |
| B�����ʴ��ͱ�������Ϊͬϵ�� |
| C�����ʴ��е���ܱȱ������� |
| D�����ʴ��������еĽ��������ᣨHOOC-COOH���������к� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
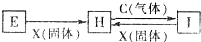 �й����ʵ�ת����ϵ��ͼ��ʾ���������ʺ�������ʡȥ����C��X���ǵ��ʣ�H��Һ��dz��ɫ��I��Һ���ػ�ɫ�������й��ж���ȷ���ǣ�������
�й����ʵ�ת����ϵ��ͼ��ʾ���������ʺ�������ʡȥ����C��X���ǵ��ʣ�H��Һ��dz��ɫ��I��Һ���ػ�ɫ�������й��ж���ȷ���ǣ�������| A��X��������ǵⵥ�� |
| B��ͼ����ʾ��Ӧ��Ϊ������ԭ��Ӧ |
| C��1mol C��E��Һ��ȫ��Ӧת�Ƶ�����ΪNA��NAΪ�����ӵ������� |
| D��E��H��I��Һ����ʱ����������ֹ��ˮ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ijУ��ѧ��ȤС���ͬѧ��һ��������Na2SO4��NaOH��Ʒ��NaOH�ĺ������вⶨ���ش��������⣺
ijУ��ѧ��ȤС���ͬѧ��һ��������Na2SO4��NaOH��Ʒ��NaOH�ĺ������вⶨ���ش��������⣺| V��HCl��/mL | 0.00 | 12.00 | 18.00 | 22.00 | 23.00 | 23.96 | 24.00 | 24.04 | 25.00 | 26.00 | 30.00 |
| pH | 13.1 | 12.6 | 12.2 | 11.7 | 11.4 | 9.9 | 7.0 | 4.0 | 2.7 | 2.4 | 1.9 |
| ָʾ�� | ��ɫ��Χ ��pH�� | ��ɫ | |
| ��ɫ | ��ɫ | ||
| ���� | 3.1��4.4 | �� | �� |
| ʯ�� | 5.0��8.0 | �� | �� |
| ��̪ | 8.2��10.0 | �� | �� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com