

| n |
| V |
| 1000��w |
| M |
| n |
| V |
| 1000��w |
| M |
| 1000��1.84��98% |
| 98 |
| n |
| V |
| n |
| V |


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ɵ��ʵķ�����һ�����й��ۼ� |
| B���ɷǽ���Ԫ����ɵĻ����ﲻһ���ǹ��ۻ����� |
| C���Ǽ��Լ�ֻ������˫ԭ�ӵ��ʷ����� |
| D�����ӻ������в����ܺ��зǼ��Լ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ǿ�������Һ�ĵ����Բ�һ�������������Һ�ĵ�����ǿ |
| B�������£���pH=3�Ĵ�����Һϡ�͵�ԭ�����10������Һ��pH=4 |
| C���������ˮ��pHС��7�����µ������� |
| D����ͨ����н����ж�����ʵ����ϡ���ɫ��ѧ��˼�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��10 mL 0.1 mol/L��ˮ��10 mL 0.1 mol/L�����ϣ�c��Cl-����c��NH4+����c��OH-����c��H+�� |
| B��10 mL 0.1 mol/L NH4Cl��Һ��5 mL 0.2 mol/L NaOH��Һ��ϣ�c��Na+��=c��Cl-����c��OH-����c��H+�� |
| C��10 mL 0.1 mol/L CH3COOH��Һ��5 mL 0.2 mol/L NaOH��Һ��ϣ�c��Na+��=c��CH3COO-����c��OH-����c��H+�� |
| D��10 mL 0.5 mol/L CH3COONa��Һ��6 mL 1 mol/L�����ϣ�c��Cl-����c��Na+����c��OH-����c��H+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��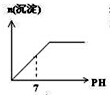 |
B��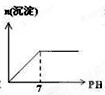 |
C��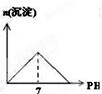 |
D��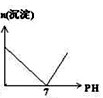 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
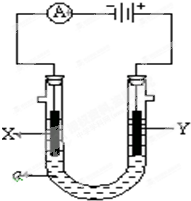 ���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�ã���ͼ��ʾһ�����أ�װ�е��Һa��X��Y������缫�壬ͨ��������ֱ����Դ��������ش��������⣺
���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�ã���ͼ��ʾһ�����أ�װ�е��Һa��X��Y������缫�壬ͨ��������ֱ����Դ��������ش��������⣺�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com