
����Ŀ���л�������F��һ����Ҫ���л��ϳ��м��壬��ϳ�·������ͼ��ʾ��

��֪����A�ĺ˴Ź�������ͼ����ʾ�����
��F�Ľṹ��ʽΪ��
��ͨ����ͬһ��̼ԭ�������������ǻ����ȶ�������ˮ�γ��ʻ���
��
��ش��������⣺
��1��A������Ϊ______________��ϵͳ����������Z�����������ŵ�������___________��
��2����Ӧ���ķ�Ӧ������__________����Ӧ���ķ�Ӧ����Ϊ__________ ��
��3��E�Ľṹ��ʽΪ_______________________��
��4��д����Ӧ���Ļ�ѧ����ʽ____________________________________________��
��5��W��Z��ͬϵ���Է���������Z��14����W��ͬ���칹������������������
���ܷ���������Ӧ���ڱ�����������ȡ�������۲���ˮ�⣬��FeCl3��Һ����ɫ�Ľṹ����
_________�֣������������칹�����˴Ź��������������ĽṹΪ____________��
��6���������ºϳ�·�ߣ������2���ȱ���Ϊ��ʼԭ�Ϻϳɱ�ȩ�ĺϳ�·�ߣ����Լ���ѡ��_____________________________________________________________��
���𰸡� 2������2������ ���ǻ���ȩ�� Ũ���ᣬ���� ������Ӧ (CH3)2CHCOOH  6
6 ![]()

��������A�ĺ˴Ź�������ͼ����ʾ����壬���A�Ļ�ѧʽ��AΪ ������B�Ļ�ѧʽ��֪��A�����ǻ�����ȥ��Ӧ����B����BΪ
������B�Ļ�ѧʽ��֪��A�����ǻ�����ȥ��Ӧ����B����BΪ![]() ��������Ϣ����B�����ӳɷ�Ӧ����C��CΪ
��������Ϣ����B�����ӳɷ�Ӧ����C��CΪ![]() ��C����������D��DΪ(CH3)2CHCHO��D��������ͭ����Һ��Ӧ���ữ����E��EΪ(CH3)2CHCOOH������F�Ľṹ
��C����������D��DΪ(CH3)2CHCHO��D��������ͭ����Һ��Ӧ���ữ����E��EΪ(CH3)2CHCOOH������F�Ľṹ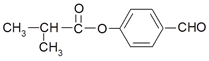 ��֪��ZΪ���ǻ�����ȩ�����YΪ
��֪��ZΪ���ǻ�����ȩ�����YΪ![]() ��XΪ
��XΪ![]() ��
��
(1)AΪ ������Ϊ2������2��������ZΪ���ǻ�����ȩ�������������з��ǻ���ȩ�����ʴ�Ϊ��2������2�����������ǻ���ȩ����
������Ϊ2������2��������ZΪ���ǻ�����ȩ�������������з��ǻ���ȩ�����ʴ�Ϊ��2������2�����������ǻ���ȩ����
(2)��Ӧ��Ϊ�ǻ�����ȥ��Ӧ����Ӧ����ΪŨ���ᣬ���ȣ���Ӧ���Ǵ��Ĵ��������ʴ�Ϊ��Ũ���ᣬ������������Ӧ��
(3)��������������EΪ(CH3)2CHCOOH���ʴ�Ϊ��(CH3)2CHCOOH��
(4)��Ӧ��Ϊ����������ȡ����Ӧ����Ӧ�Ļ�ѧ����ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
(5)W��Z(���ǻ�����ȩ)��ͬϵ���Է���������Z��14�����ܷ���������Ӧ��˵������ȩ�����ڱ�����������ȡ�������۲���ˮ�⣬��FeCl3��Һ����ɫ��˵��û�з��ǻ��������������������У������Ϻ���1��ȩ����1����CH2OH����3�ֽṹ�������Ϻ���1��ȩ����1����O CH3����3�ֽṹ����6�֣����к˴Ź��������������ĽṹΪ![]() ���ʴ�Ϊ��6��
���ʴ�Ϊ��6��![]() ��
��
(6)��2���ȱ���Ϊԭ�Ϻϳɱ�ȩ(CH3CH2CHO)��ֻ��Ҫ����ԭ����ȥ��Ȼ�������Ϣ����̼̼˫���������ǻ������������ɣ��ϳ�·��Ϊ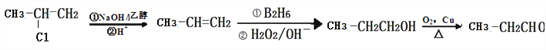 ���ʴ�Ϊ��
���ʴ�Ϊ��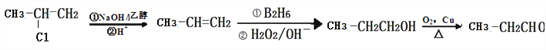 ��
��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й��ڹ輰�仯�����˵���У���ȷ���ǣ� ��
A.���dz��õİ뵼����ϣ�������������άB.��������������������ʲ����κ��ᷴӦ
C.������һ�ֶ�Ԫ���ᣬ������ǿ��̼��D.������ͨ��������Ҫԭ���Ǵ��ʯ��ʯ��ʯӢ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��POCl3�㷺����Ⱦ�ϵȹ�ҵ��ij��ѧѧϰС������������о������ɷֵ�����굣�װ�üף��ϳ�PC13������ȡPCl3�������Ʊ�POCl3��
��֪����1��PCl3���۵�Ϊ-112�棬�е�Ϊ75.5�棬��ˮ����H3PO3��HCl��
��2��2PCl3+O2==2POCl3��
��ʵ����Ʊ�PCl3
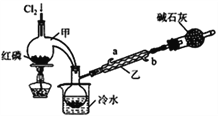
��1��ʵ�����Ʊ�Cl2��ԭ����________________________��
��2����ʯ�ҵ����ó��˴���β�����________________________��
��3��װ����������ˮ��_____��ѡ��a��b�����롣
��ʵ����Ʊ�POCl3
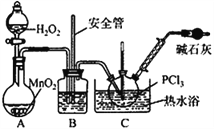
��4��ʵ���ҳ��������Լ�ƿ����H2O2�����ס�������װ���е�___________���������ƣ�Ŀ����һ�µġ�
��5��C�з�Ӧ�¶ȿ�����60~65�棬��ԭ����________________________��
��ʵ��ⶨPOCl3����
��ȷ��ȡ30.70gPOC13��Ʒ������ʢ��60.00mL����ˮ��ˮ��ƿ��ҡ������ȫˮ�⣻
�ڽ�ˮ��Һ���100.00mL��Һ��ȡ10.00mL��Һ����ƿ�У�
�ۼ���10.00mL3.200mol/LAgNO3����Һ����������������������ҡ����ʹ�������汻�л��︲�ǣ�
����Fe3+ Ϊָʾ������0.2000mol/LKSCN ��Һ�ζ�������AgNO3��Һ���ﵽ�ζ��յ�ʱ����ȥ10.00 mL KSCN ��Һ��
��֪��Ag++SCN-==AgSCN�� Ksp(AgCl)>Ksp(AgSCN )��
��6��POC13ˮ��Ļ�ѧ��Ӧ����ʽΪ________________________��
��7���ζ��յ������Ϊ____________�������������dz�����Ŀ����________________________��
��8����Ӧ��POC13�İٷֺ���Ϊ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����������ʹ��ǰ�������Ƿ�©ˮ����_________(�����)��
A������ƿ B��������ƿ C����Һ©�� D����Ͳ E��������
��2��ʵ������Na2CO3��10H2O��������0.5mol/L��Na2CO3��Һ970mL��Ӧѡ�õ�����ƿ�Ĺ��___________��Ӧ�������ٿ�Na2CO3��10H2O����_____��
��3��ijѧ������10mol��L��1Ũ���������ˮ����500mL���ʵ���Ũ��Ϊ5mol��L��1��ϡ���ᡣ������ҪŨ�������Ϊ___mL�����ƹ�������ȷ�IJ���˳���ǣ���ĸ��ʾ��ÿ����ĸֻ����һ�Σ�_________
A.������ˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ����
B.����Ͳ��ȡ����Ũ���ᣬ�ز����������ձ��У��ټ�������ˮ���ò���������������ʹ���Ͼ���
C.������ȴ�������ز�����ע��500mL����ƿ��
D.������ƿ�ǽ�����ҡ��
E.���ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�����
F.����������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1��2cm��
��4������һ�������һ�����ʵ���Ũ�ȵ���Һ,ʵ����ƫ��Ӱ�����_______
A.����ƿ��ԭ������ˮ B.�ܽ������ձ�δϴ�� C.����ʱ���ӹ۲�̶��� D.����ʱ���ӹ۲�̶���
��5����ͼ��ijͬѧ��ʵ�������Ƹ�NaCl��Һ�Ĺ���ʾ��ͼ�������д������______(��������)��


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Է�������ΪM����̬������V L(��״��)������m gˮ�У��õ���������Ϊw����Һ�����ʵ���Ũ��Ϊc mol��L��1���ܶ�Ϊ�� g��cm��3��������˵������ȷ����(����)
A. ��Է�������M��22.4mw/(1��w)V B. ���ʵ���Ũ��c��1000��V/(MV+22.4m)
C. ��Һ����������w��MV/22.4m D. ��Һ�ܶȦѣ�cM/1000w
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������֯��WHO����ClO2��ΪA����Ч��ȫ���������������ʳƷ���ʡ�����ˮ�����ȷ����й㷺Ӧ�á�ClO2����
A.��B.��C.������D.��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)�����������ʣ���NA��HCl ��24gCH4 ��4.816��1023��H2O2���� ��10.8mLˮ���ܶ�Ϊ1g��cm��3����
�����������������������ɶൽ�ٵ�˳���ǣ�_________________________________��
����������������ԭ�����ɶൽ�ٵ�˳���ǣ�_________________________________��
����������������H�ɶൽ�ٵ�˳���ǣ�_____________________________________��
�����������ʵ������ɶൽ�ٵ�˳���ǣ�_____________________________________��
(2)H2O��CO2��������Ϊ18:22����H2O��CO2�����ʵ���֮��Ϊ________�����Ӹ�����Ϊ_______��ԭ�Ӹ�����Ϊ__________��������ԭ�Ӹ�����Ϊ___________��
(3)�ڱ�״���£���������O3��CO2�Ƚϣ��ܶȱ�Ϊ________��������֮��Ϊ_________��ԭ����֮��______�������Ϊ_______�����ʵ���֮��__________��
(4)CH4��H2�Ļ�����壬�������ٷֺ����ֱ�Ϊ80%��20%�����������ƽ����Է�������Ϊ__________��
(5)��״���£�ij�����ܶ�Ϊ1.96g/L����������Ħ������Ϊ_____������������
(6)��100��ijNaOH��Һ���ܶ���1.22g/cm3������Ũ����������Һ50mlʱ�����ʵ���Ũ����8mol/L��ԭ��Һ�����ʵ���Ũ���� _________________��
(7)��֪Na2S��Na2SO3��Na2SO4���������Ԫ�ص���������Ϊa%������Ԫ�ص���������Ϊ_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������̪���ҹ������з���һ���������Ƽ���ȱѪ���������ҩ���ϳɶ���̪(J)��һ��·�����£�


��1��A��������_______________��A�����������_____��ԭ�ӹ�ƽ�档
��2��B����A�Ļ�ѧ����ʽ______________________��
��3��D����E�ķ�Ӧ����Ϊ_________���Լ�a��_________��
��4��F�Ľṹ��ʽ_____________________��
��5��J��һ�����������г����������һ����Ԫ����д��H����J�Ļ�ѧ����ʽ_____��ע����Ӧ��������
��6��![]() ��X��ͬ���칹���У����ܷ���������Ӧ���������Ȼ�����Һ������ɫ��Ӧ����������������X��ͬ���칹�干��______�֣�д�����к˴Ź����������������շ�Ľṹ��ʽ___________________��
��X��ͬ���칹���У����ܷ���������Ӧ���������Ȼ�����Һ������ɫ��Ӧ����������������X��ͬ���칹�干��______�֣�д�����к˴Ź����������������շ�Ľṹ��ʽ___________________��
��7������������Ϣ����ѧ֪ʶ��д���Լ���ͻ�����DΪԭ�ϣ��ϳ� ![]() ��·������ͼ____________________�������Լ���ѡ����
��·������ͼ____________________�������Լ���ѡ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��TiO2��һ�����������İ뵼������������Ч�������л���Ⱦ�������ȩ���ױ���)�ͺ���������(��NH3��CN������ת��ΪCO2��N2��С�������ʡ�
��1��Ti��̬��������Ų�ʽΪ________________��
��2����ȩHCHO���ӿռ乹��Ϊ_____��������̼ԭ�ӹ���ӻ�����Ϊ_____���м��ͦҼ��ĸ���֮��Ϊ____��
��3��������������ˮ������Ϊ����ˮ�ķ��Ӿ���_________������Ϊ___________��
��4���ױ��������ܹ���ƽ���ԭ�����Ϊ____�����������ױ�±�ؼӳɣ����Ƚ����ױ�±��ȡ�������ϵ��⣬ԭ����___________________��
��5����CN������ˮ���Լ�������NaClO�Ȱ�CN������ΪCNO����Ȼ���������������ٽ�CNO������Ϊ����Ⱦ�����塣��д����CNO����Ϊ�ȵ��������______���ӻ����ӣ�дһ�֣���
��6��Ti[(CN)4]2-��Ti2+��CN-��Cԭ���γ���λ���������ǿռ乹�ͣ�Ti[(CN)4]2-�Ľṹ�ɱ�ʾΪ_____________________��
��7��Ti��ij�������CaO��������γ������εľ����ṹ��ͼ��ʾ��Ti4+λ��������Ķ��㣬Ca2+ ��������������ģ����þ����У�Ti4+����Χ____ ��O2-����ڣ����þ������ܶ�Ϊdg/cm3���������ļ���Ϊ______pm ���ô�NA�Ĵ���ʽ��ʾ����

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com