
| ��ѧʽ | CH3COOH | H2CO3 | HClO |
| ����ƽ�ⳣ�� | 1.7��10-5 | K1=4.3��10-7 K2=5.6��10-11 | 3.0��10-8 |
���� ��1����ĵ���ƽ�ⳣ��Խ�������̶�Խ��������Խǿ��
��2����ĵ���ƽ�ⳣ��Խ�������Ӧ���������ˮ��̶�ԽС���������Ӧ��������ӽ��������Ũ��ԽС��
��3����ĵ���ƽ�ⳣ��Խ�������Ӧ���������ˮ��̶�ԽС������ͬŨ�ȵ�������ҺpHԽС��
��� �⣺��1����ĵ���ƽ�ⳣ��Խ�������̶�Խ��������Խǿ�����ݱ�������֪����ĵ���̶ȴ�С˳����CH3COOH��H2CO3��HClO��HCO3-����������ǿ��˳����CH3COOH��H2CO3��HClO��HCO3-���ʴ�Ϊ��CH3COOH��H2CO3��HClO��
��2����ĵ���ƽ�ⳣ��Խ�������Ӧ���������ˮ��̶�ԽС���������Ӧ��������ӽ��������Ũ��ԽС�����ݣ�1��֪�����ǿ��˳����CH3COOH��H2CO3��HClO��HCO3-�����������ˮ��̶ȴ�С˳����CO32-��ClO-��HCO3-��CH3COO-������������ӽ������������С˳����CO32-��ClO-��HCO3-��CH3COO-���ʴ�Ϊ��CO32-��ClO-��HCO3-��CH3COO-��
��3����ĵ���ƽ�ⳣ��Խ�������Ӧ���������ˮ��̶�ԽС������ͬŨ�ȵ�������ҺpHԽС�����ݣ�1��֪�����ǿ��˳����CH3COOH��H2CO3��HClO��HCO3-�����������ˮ��̶ȴ�С˳����CO32-��ClO-��HCO3-��CH3COO-��������ͬŨ�ȵ������ҺpH��С˳����Na2CO3��NaClO��CH3COONa���ʴ�Ϊ��Na2CO3��NaClO��CH3COONa��
���� ���⿼��������ʵĵ��������ˮ�⣬Ϊ��Ƶ���㣬��ȷ����ƽ�ⳣ�����������ǿ�����������ˮ��̶ȴ�С��ϵ�ǽⱾ��ؼ���ע�⣺CO32-��Ӧ������HCO3-������H2CO3��Ϊ�״��㣮


 ��ĩ���ƾ�ϵ�д�
��ĩ���ƾ�ϵ�д� ���ɿ��ñ���ϵ�д�
���ɿ��ñ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ������и߶���9�µ��л�ѧ�Ծ��������棩 ���ͣ�ѡ����
��Щ��ѧ��Ӧ��Ӧ���ʺ����Ҹ���Ӧ�϶࣬�ⶨ��Щ��Ӧ���ʱ�����ã� ��
A����˹���� B����������ԭ��
C�������ӵ����� D�������غ㶨��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Һ��pH | B�� | ����ĵ���ƽ�ⳣ�� | ||
| C�� | ��Һ�ĵ������� | D�� | ����ĵ���̶� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
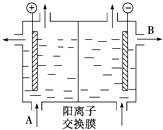 ��Ҫ��������и�С��
��Ҫ��������и�С���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | HClO2 | HF | HCN | H2S |
| Ka | 1��10-2 | 6.3��10-4 | 4.9��10-10 | K1=9.1��10-8K2=1.1��10-12 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ��1��ʵ�鲽�� | �й����� |
| �ټ�������Na2SO4������ | ��Ҫ����Na2SO4������Ϊ2.8g |
| �ڳ���Na2SO4���� | ������Ҫ�õ�����Ҫ�����ǣ�������ƽ |
| �۽�Na2SO4����100mL�ձ��У�����������ˮ | �ò�������������ȫ�ܽ⣬��ȴ������ |
| �ܽ��ձ�����Һת��������A�У��Ѽ�鲻©ˮ�� | ����A��100mL����ƿ�� |
| ��ϴ���ձ���ת�ƣ����� | |
| ��ҡ�ȡ�װƿ�����ϱ�ǩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com