
���� ��1��ˮ�����ӻ�����KW=10-14��pH=9��Ba��OH��2��Һ������������Ũ��Ϊ0.00001mol/L��pH=4��H2SO4��Һ��������Ũ��Ϊ0.0001mol/L�������û��ҺΪ���ԣ����ǡ����ȫ��Ӧ�������ӵ����ʵ����������������ӵ����ʵ������ݴ���ʽ�����Ba��OH��2 ��Һ�� H2SO4 ��Һ������ȵ�ֵ������Q=c��Ba2+��•c��SO42-��������Һ��c��SO42-����1.0��10-5mol•L-1����Ӧ������Һ�� c��Ba2+����ֵ��
��2��CH3COOH��Һ��������CH3COONa����ʱƽ�����淴Ӧ�����ƶ�������̶ȼ�С�����ǵ���ƽ�ⳣ��ֻ���¶��йأ��¶Ȳ��䣬K���䣻
��3��CH3COONH4�����������Σ������£������NH3•H2O�ĵ���ƽ�ⳣ����Ϊ1.74��10-5˵��CH3COO-��NH4+ˮ��̶���ͬ����Һ�����ԣ���H2CO3��������CH3COOH��HCO3-��ˮ��̶ȴ���CH3COO-��NH3•H2O��CH3COOH��ǿ���൱��������ʣ�H2CO3����̶�����NH3•H2O���ݴ˷������
��� �⣺��1�������£�ˮ�����ӻ�����KW=10-14��pH=9��Ba��OH��2��Һ������������Ũ��Ϊ0.00001mol/L��pH=4��H2SO4��Һ��������Ũ��Ϊ0.0001mol/L�������û��ҺΪ���ԣ����ǡ����ȫ��Ӧ�����У�n��H+��=n��OH-������Ba��OH��2 ��Һ�� H2SO4 ��Һ������ֱ�ΪaL��bL������0.00001mol/L��aL=0.0001mol/L��bL��a��b=10��1��
Ba2+��aq��+SO42-��aq��?BaSO4��s���������£�Ksp��BaSO4��=1.1��10-10����ʹ��Һ��c��SO42-����1.0��10-5mol•L-1��Q=c��Ba2+��•c��SO42-����Ӧ������Һ�� c��Ba2+����$\frac{{K}_{sp}��BaS{O}_{4}��}{1.0��1{0}^{-5}mol/L}$=$\frac{1.1��1{0}^{-10}}{1.0��1{0}^{-5}}$=1.1��10-5mol•L-1��
�ʴ�Ϊ��10��1��1.1��10-5��
��2��CH3COOH��Һ�д��ڵ���ƽ�⣺CH3COOH?CH3COO-+H+��1L 0.1mol•L-1CH3COOH��Һ�к�0.1molCH3COOH����Һ�м���0.1molCH3COONa����n��CH3COO-������ƽ���淴Ӧ�����ƶ�������ƽ�ⳣ��ֻ���¶��йأ��¶Ȳ��䣬K���䣬
�ʴ�Ϊ���棻���䣻
��3�������£������NH3•H2O�ĵ���ƽ�ⳣ����Ϊ1.74��10-5��NH3•H2O��CH3COOH��ǿ���൱��������ʣ���CH3COONH4��Һ�����ԣ���H2CO3��������CH3COOH��HCO3-��ˮ��̶ȴ���CH3COO-������NH4HCO3��Һ�ʼ��ԣ�̼�����Ϊǿ����ʣ���ȫ�������ɰ������Ӻ�̼��������ӣ�NH4HCO3=NH4++HCO3-��NH3•H2O��CH3COOH��ǿ���൱��������ʣ�H2CO3����̶�����NH3•H2O��HCO3-��ˮ��̶ȴ���NH4+������NH4HCO3��Һ�����ʵ���Ũ������������NH4+��
�ʴ�Ϊ���У��NH4+��
���� ���⿼��PH�ļ��㡢�����ܽ�ƽ����㡢����ˮ�⣬�ۺϿ���ѧ����ѧ֪ʶ��Ӧ�������ͷ��������������Ϊ�߿��������ͣ�ע���������ˮ���������ʵĵ����ص㣮
ע�������ӻ�Q���ڣ�Ksp��BaSO4������������������֮�����ޣ���Ŀ�Ѷ��еȣ�


 ��ѧ����ͬ����ϰϵ�д�
��ѧ����ͬ����ϰϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 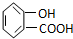 | B�� | CH3CH2=CHCOOH | C�� | 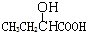 | D�� | 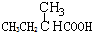 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
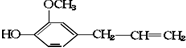 ����ܾ��е������ǣ����ܷ����ӳɷ�Ӧ������ʹ���Ը��������Һ��ɫ�����ܷ���ȡ����Ӧ�����������Ȼ���������ɫ��Ӧ�����ܷ����кͷ�Ӧ�����ܷ�����ȥ��Ӧ��������
����ܾ��е������ǣ����ܷ����ӳɷ�Ӧ������ʹ���Ը��������Һ��ɫ�����ܷ���ȡ����Ӧ�����������Ȼ���������ɫ��Ӧ�����ܷ����кͷ�Ӧ�����ܷ�����ȥ��Ӧ��������| A�� | ȫ�� | B�� | ���٢ڢۢ� | C�� | �����ⶼ�� | D�� | ���ܢ��ⶼ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NH3��NH4Cl | B�� | HCl��SiO2 | C�� | KCl��K | D�� | CO2��H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 11.11 | B�� | 22.22 | C�� | 30.00 | D�� | 32.00 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | �÷�ӦΪ���ȷ�Ӧ | |
| B�� | �÷�Ӧ��ΪBa��OH��2•8H2O��NH4Cl����ķ�Ӧ | |
| C�� | �÷�Ӧ��Ϊ�����������е�ȼ�շ�Ӧ | |
| D�� | �÷�Ӧֻ���ڼ��������²��ܽ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  �������� �������� | B�� |  ��1-��-1-���� ��1-��-1-���� | ||
| C�� |  4һ��-���� 4һ��-���� | D�� |  2-�һ�-1-��ϩ 2-�һ�-1-��ϩ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com