
����Ŀ����.������8�־��壬����Żش��������⣺
A��ˮ�� B�������� C������ D����̬� E���Ȼ�� F���� G�����ʯ
��1������ԭ�Ӿ���Ļ�������___���������Ӿ������___��������ѧ���ķ��Ӿ�����____��
��2���ɼ��Է��ӹ��ɵľ�����___�����й��ۼ������Ӿ�����___�����ڷ��Ӿ���ĵ�����____��
��3�������ڴ��ڻ�ѧ�����������ۻ�ʱ����ѧ���������仯����___�������ۻ�����˷����ۼ�����____��
��.���мס��ҡ����������־���(��ͼ��ʾ)������֪��������A��B�����Ӹ�����Ϊ___���Ҿ���Ļ�ѧʽΪ___��������Ļ�ѧʽΪ____��������Ļ�ѧʽΪ____��

���𰸡�A E D B E CD BC AG 1��1 C2D EF XY3Z
��������
I.�������ʵĹ���������֮����������жϾ������ͣ����ݹ������ʵ�Ԫ���ж��Ƿ����ڻ��������һ��Ԫ�ع��ɵ��������ڵ��ʣ����������ϵ�Ԫ�ع��ɵ��������ڻ�����������ɵ�ԭ�ӷ��ӹ��ɣ����������ڷ��Ӿ��壬��ѧ����ͬ��Ԫ�ص�ԭ��֮���γɵĹ��ۼ����ڷǼ��Թ��ۼ�����ͬ��Ԫ�ص�ԭ���γɵĻ�ѧ�����ڼ��Թ��ۼ������������ۻ�ʱ�Ƿ�����ѧ�仯�ж��Ƿ���ѻ�ѧ����
II.�þ�̯�����ж����ʵĻ�ѧʽ��Ȼ����ݹ��������������ж�����Ӧ�Ļ�ѧʽ��
��.(1)ˮ�������ʯ������ԭ��ͨ�����ۼ��γɵ�������״�ṹ����˶��߶�����ԭ�Ӿ��壬����ˮ����ѧ�ɷ�Ϊ�������裬��Si��O����Ԫ����ɣ����ڻ�������ʯ���Ԫ��ֻ��CԪ��һ��Ԫ�أ����ڵ��ʣ��Ȼ������NH4+��Cl-ͨ�����Ӽ���϶��γɵ����ӻ�����ڹ���ʱ�������Ӿ��壻�����ᡢ���ס���̬벶����ɷ���ͨ�����Ӽ���������϶��γɵķ��Ӿ��壬����ϡ������벷����в����ڻ�ѧ�����Dz�����ѧ���ķ��Ӿ��塣��������ԭ�Ӿ���Ļ������������A���������Ӿ�������������E��������ѧ���ķ��Ӿ��������D��
(2)���������ɼ��Է��ӹ��ɵķ��Ӿ��壻�Ȼ���к������Ӽ����ۼ��������й��ۼ������Ӿ������Ȼ�泥�����̬벾��Ƿ��Ӿ��壬�������Ԫ����ֻ��һ��Ԫ�أ������ڵ��ʣ����ɼ��Է��ӹ��ɵľ��������B�����й��ۼ������Ӿ��������E�����ڷ��Ӿ���ĵ��������CD��
(3)������Ͱ����Ǻ��й��ۼ��ķ��Ӿ��壬�ۻ�ʱ�ƻ����Ӽ��������������ƻ������ڵĹ��ۼ���ˮ���ͽ��ʯ����ԭ��֮��ͨ�����ۼ�����γɵ�ԭ�Ӿ��壬�ۻ�ʱ�ƻ����ۼ������Է����ڴ��ڻ�ѧ�����������ۻ�ʱ����ѧ���������仯�����������BC�������ۻ�����˷����ۼ������������AG��
��.�þ�̯�����ж����ʵĻ�ѧʽ�����У�
�����к�A����1����B����8��![]() =1����������Ŀ��Ϊ1��1��
=1����������Ŀ��Ϊ1��1��
�Ҿ����к�C����1����D����4��![]() =
=![]() ����C��D������Ŀ��Ϊ1��
����C��D������Ŀ��Ϊ1��![]() =2��1������Ҿ��廯ѧʽΪC2D��
=2��1������Ҿ��廯ѧʽΪC2D��
�������к�E���ӡ�F������Ŀ��Ϊ4��![]() =
=![]() ������E��F������Ŀ��Ϊ
������E��F������Ŀ��Ϊ![]() ��
��![]() =1��1���ʱ����廯ѧʽΪEF��
=1��1���ʱ����廯ѧʽΪEF��
�������к�X����1����Y����6��![]() =3����Z����8��
=3����Z����8��![]() =1����������X��Y��Z������Ŀ��Ϊ1��3��1�����Զ����廯ѧʽΪXY3Z��
=1����������X��Y��Z������Ŀ��Ϊ1��3��1�����Զ����廯ѧʽΪXY3Z��


 �����������һ��һ��ϵ�д�
�����������һ��һ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ʵ��˵��������������ʵ��� ( )
A. ������ʹʯ����Һ���
B. ![]() ʱ.
ʱ.![]() �����pHԼΪ3
�����pHԼΪ3
C. ������м�������������Һ����ҺpH����
D. ��������̼��Ʒ�Ӧ����![]() ����
����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���0.1 mol��L��1 NaOH��Һ�ζ�10 mL 0.1 mol��L��1 H2X��Һ����Һ��pH��NaOH��Һ�������ϵ��ͼ��ʾ������˵������ȷ����

A.ˮ���������c(OH��)��D�㣾B��
B.C����ڹ�ϵʽ��c(Na��)��c(HX��)��c(X2��)��c(H��)
C.B�㣺c(HX��)��c(H��)��c(X2��)��c(H2X)
D.A����Һ�м�������ˮ��![]() ����
����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NaOH��Na2CO3�����Һ�еμ�0.1mol/Lϡ���ᣬCO2���������������������(V)�Ĺ�ϵ��ͼ��ʾ.�����ж���ȷ����(
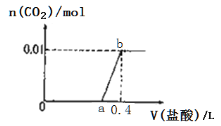
A. ��0~a��Χ�ڣ�ֻ�����кͷ�Ӧ
B. ԭ�����Һ��NaOH��Na2CO3�����ʵ���֮��Ϊ1:2
C. a = 0.3
D. ab�η�����Ӧ�����ӷ���ʽΪ:CO32-+2H+=H2O+CO2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ����NaCl��Na2CO3.10H2O��NaHCO3�Ļ���ijͬѧ�����ͼ��ʾ��ʵ��װ�ã�ͨ��������Ӧǰ��CO2��H2O����������ȷ���û�����и���ֵ�����������
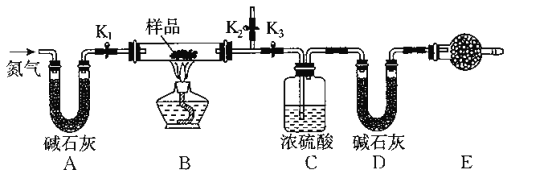
��1��ʵ�鲽��
�ٰ���ͼ���г�����δ��������װ��ʵ��װ�ú����Ƚ��еIJ�����_______________��
�ڳ�ȡ��Ʒ�����������Ӳ�ʲ������У�����װŨ�����ϴ��ƿC��������װ��ʯ�ҵ�U�ι�D��������
�۴���K1 K2���ر�K3���������뵪�������ӣ���Ŀ����_____________________��
�ܹرջ���K1 K2����K3����ȼ�ƾ��Ƽ��ȣ������ٲ�������Ϊֹ��
�ݴ���K1���������뵪�������ӣ�Ȼ�����װ�ã��ٴγ���ϴ��ƿC��������U�ι�D��������
��2�����ڸ�ʵ�鷽�����ش��������⡣
�������ȷ�Ӧ����������Բⶨ�����Ӱ����__________________________��
��E���������ʢ�ŵ�ҩƷ��_________����������_________________________�����ʵ����û�и�װ�ã���ᵼ�²������NaHCO3����������________(����ƫ��������ƫС��������Ӱ����)��
������Ʒ����Ϊwg����Ӧ��C��Dװ�����ӵ������ֱ�Ϊmg��ng����������Na2CO3.10H20����������Ϊ________(�ú�w��m��n�Ĵ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��0.1molij��̬���������ȫȼ������13.2gCO2��4.5g H2O�������з��ϴ������Ļ����ѡ��Ϊ
A. C2H4��C4H6B. C2H2��C4H4C. C2H2��C3H8D. C2H4��C4H8
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���л���Ľṹ����������ʽ����ʾ��CH3��CH��CH��CH3�ɼ�дΪ ![]() ���л���X�ļ���ʽΪ��
���л���X�ļ���ʽΪ��

��1���л���X�ķ���ʽ___________________
��2���л���Y��X��ͬ���칹�壬���ڷ�������д��Y�Ľṹ��ʽ _______________________________________________________
��3��X��������H2��һ�������·�Ӧ�����ɻ�״�ı�����Z��Z��һ�ȴ�����_________�֡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ����������װ����ȡ������������������ʵ�顣�ش��������⣺

��1��A��ʢ��Ũ���ᣬB��ʢ��MnO2��д����Ӧ�Ļ�ѧ����ʽ_______��
��2��D�з���ŨH2SO4����Ŀ����_____________________________��
��3��E��Ϊ��ɫ�ɲ�����F��Ϊ��ɫʪ�������ɹ۲쵽��������___________���Ա�E��F������IJ���ɵó��Ľ��ۼ�������____________________��
��4��G����������____________________________________��
��5������H��β������װ��ͼ��ע���Լ�____________��
��6����ͥ�г�������Һ����Ҫ�ɷ�NaClO�������飨��Ҫ�ɷ����ᣩ���������ijƷ������Һ��װ��˵����ͼ��
ע�����
1����Ʒ����֯Ʒ��Ư����ɫ���ã��Խ�����Ʒ�и�ʴ���á�
2���ܷⱣ�棬����������ͬʱʹ�á�
3��������Ϊһ�ꡣ
��������ͬʱʹ����������ж���������д����Ӧ�����ӷ���ʽ________��
��7��������һ����Ϊ����ˮ�����IJ�ƷҲ�ܶ�����ˮ���п��ٵ�ɱ��������ҩ��ͨ�����������㡣�������Ⱦ�Cl2Na(NCO)3����ˮ��Ӧ�����ɴ�������ɱ���������ã������Ӻ��ڲ���������ƣ�Na2SO3���ܳ����ɽ�ˮ�е����ȣ�������ȣ���ȥ���������ƽ�ˮ�ж���������ȥ�����ӷ�Ӧ����ʽΪ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����֪����ͨ���ȵĿ�������Һ�лᷢ�����·�Ӧ��3Cl2+6NaOH=5NaCl+NaClO3+3H2O��Ӧ�л�ԭ����___���ѧʽ�����Ѵ˷�Ӧ��д�����ӷ���ʽ��___��
��2���á�˫���š���ʾ����������ԭ��Ӧ�е���ת�Ƶķ������Ŀ��3Cl2+6NaOH=5NaCl +NaClO3+3H2O��___
��3��������1mol��NaClO3��ת�Ƶĵ�������___������Ӧ��ת����2.5mol�ĵ��ӣ������ı����Cl2�������___��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com