
�л������������������ܲ��ɷ֣������е�һЩ���ⳣ�漰��ѧ֪ʶ��
(1)�����м���ʳƷ��
�ٻ����������������������ҪӪ������Ϊ________(����ࡱ����֬�������ʡ�)��
�ڳԷ�ʱ������һ�����о�����ζ������Ϊ���۷�����________��Ӧ(ѡ������ѡ���)��
A���ֽ⡡����B��ˮ�⡡����C���ѽ�
(2)���ճ������У����������������________��
A����ȼ�շ�����ë֯Ʒ����֯Ʒ
B���ô���ϴ�ӹ����ϵ�����
C��������ζ�ķ�������ƺ��״�
D���õ�����Һ����ӵ�ʳ�κͲ������ʳ��
 ��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(14��)��������A(C11H8O4)������������Һ�м��ȷ�Ӧ�����ữ�ɵõ�������B��C��
�ش��������⣺
��B�ķ���ʽΪC2H4O2��������ֻ��һ�������š���B�Ľṹ��ʽ��________��B���Ҵ���Ũ������¼��ȷ�Ӧ����D���÷�Ӧ�Ļ�ѧ����ʽ��________________________���÷�Ӧ��������________��д�����־���ȩ������CHO����B��ͬ���칹��Ľṹ��ʽ________________________________��
��C�Ƿ����廯�����Է�������Ϊ180����̼����������Ϊ60.0%�������������Ϊ4.44%������Ϊ������C�ķ���ʽ��________��
����֪C�ı�����������ȡ����������һ��ȡ������֧�����Һ�����ʹ������Ȼ�̼��Һ��ɫ�Ĺ����ż�����̼��������Һ��Ӧ�ų�����Ĺ����ţ����ȡ�����ϵĹ�����������________����������ȡ������ͬ���ֱ�λ�ڸ�ȡ��������λ�Ͷ�λ����C�Ľṹ��ʽ��________________�����ֻ�仯����C����������ȡ������λ�ã�����Եõ�________��C��ͬ���칹�塣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ҫ��������з�Ӧ�Ļ�ѧ����ʽ����ע����ط�Ӧ�ķ�Ӧ���͡�
(1)����������������������������������
����ʽ__________________________________________________________��
��Ӧ����________________________________________________________��
(2)��������H2�������ɼ�����
����ʽ___________________________________________________________��
��Ӧ����________________________________________________________��
(3)������������Cu(OH)2����Һ�������ɺ�ɫ����
����ʽ__________________________________________________________��
��Ӧ����_________________________________________________________��
(4)��������������ˮ��
����ʽ__________________________________________________________��
��Ӧ����________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧ�ҷ���ijҩ��M��������Ѫ�ܼ�������Ϊ�������������ͷų�һ�֡���ʹ���ӡ�D����������D�������ڵ�����ԭ����Ϊ�������ٻ���ŵ������ѧ��ҽѧ����
��ش��������⣺
(1)��֪M����Է�������Ϊ227����C��H��O��N����Ԫ����ɣ�C��H��N��������������Ϊ15.86%��2.20%��18.50%����M�ķ���ʽ��____________��D��˫ԭ�ӷ��ӣ���Է�������Ϊ30����D�ķ���ʽΪ____________________��
(2)��֬A������;���ɵõ�M��
�Ԣڵ���ʾ��
C2H5OH��HO�� NO2 C2H5O�� NO2��H2O
C2H5O�� NO2��H2O
���� ��������
��Ӧ�ٵĻ�ѧ����ʽ��_________________________________________��
��Ӧ�ڵĻ�ѧ����ʽ��_______________________________________��
(3)C��B��������һ�������·�Ӧ���ɵĻ������Է�������Ϊ134��д��C���п��ܵĽṹ��ʽ__________________________________________��
(4)����0.1 mol B�������Ľ����Ʒ�Ӧ����������________ g�����ơ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij�л���A��ѧʽΪCxHyOz,15 g A��ȫȼ�տ�����22 g CO2��9 g H2O������
��1�����л�������ʽ________��
��2����A����Է�������Ϊ60�Һ�Na2CO3���������ų���A�ʹ��ܷ���������Ӧ����A�Ľṹ��ʽΪ________��
��3����A����Է�������Ϊ60�����ӷ���ˮ����ζ��Һ�壬�ܷ���ˮ�ⷴӦ������ṹ��ʽΪ________________��
��4����A���ӽṹ�к���6��̼ԭ�ӣ����ж�Ԫ����ȩ�����ʣ�����ṹ��ʽΪ_____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����12�֣��������������ʣ��� CH3OH ��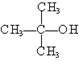
��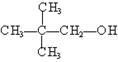 ��
��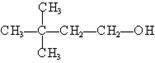 ��
��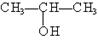
��1���ܷ�����ȥ��Ӧ����ϩ������ ��������ţ���ͬ��������ȥ���������� ���ɴ˿����Ƴ����״���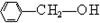 �� ����ܡ����ܡ���������ȥ��Ӧ��
�� ����ܡ����ܡ���������ȥ��Ӧ��
��2������ͭ�Ĵ������£�������������������ȩ����ŷֱ��� ����д���ܱ�������ͪ�ķ�Ӧ����ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��5�֣������dzµ��㡱��������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ��������ͼ��ʾ��װ����ȡ�����������ش��������⣺
��1��д����ȡ���������Ļ�ѧ��Ӧ����ʽ��
______________________________________________________________
��2������̼������Һ����Ҫ������_____________________________________________��
��3����Ҫ���Ƶõ������������������Ӧ���õ�ʵ�������_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������ʾ��������ζ�������������£�
| ������� | ������ |
| ���� | 1 |
| �Ǿ� | 500��700 |
| ��˹���� | 180��200 |
| Ŧ�� | 7000��13000 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪�л���A��B��ֻ��C��H��O����Ԫ�ء�
(1)A����Է�������Ϊ62��A������������D��D������������E��A��E��һ�������·�Ӧ������һ�ֻ�״������F��
��A�ķ���ʽΪ________���ṹ��ʽΪ________��A��E��Ӧ����F�ķ�Ӧ����Ϊ________��
(2)���л���B����ɡ��ṹ���з����ⶨ����ʵ�������£�
����ȫȼ��166 mg�л���B���õ�352 mg CO2��54 mg H2O��
�ڲ��B�ĺ˴Ź���������2���壬���������ʾ�ṹ�к��������Ȼ���
��B����Է���������100��200֮�䡣
��B����Է�������Ϊ________���ṹ��ʽΪ________��
(3)A��B��һ�������·�Ӧ������һ�ֳ����ϳ���ά���÷�Ӧ�Ļ�ѧ����ʽΪ________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com