
����Ŀ�������й���Һ������Ũ�ȵĹ�ϵʽ�У���ȷ����
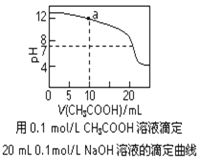
A. pH��ͬ�Ģ�CH3COONa����NaHCO3����Na2CO3������Һ�е�c(Na��)���ۣ��ڣ���
B. 0.1mol��L��1ij��Ԫ����ǿ����NaHA��Һ�У�c(Na+)=2c(A2-)��c(HA-)��c(H2A)
C. ͼ��pH��7ʱ��c(Na��)��c(CH3COO��) ��c(OH��)��c(H��)
D. ͼ��a����Һ�и�����Ũ�ȵĹ�ϵ�ǣ�c(OH��)��c(H��)��c(CH3COO��)��2c(CH3COOH)
���𰸡�D
��������
A��CH3COONa��NaHCO3��Na2CO3ˮ��̶������������Ե�������Һ��pH��ͬʱ�������ʵ���Ũ���ɴ�С��˳���Ǣۣ��ڣ��٣���A����
B�����������غ㣬0.1mol��L��1ij��Ԫ����ǿ����NaHA��Һ�У�c(Na+)=c(A2-)��c(HA-)��c(H2A)����B����
C�����ݵ���أ�ͼ��pH��7ʱ��c(Na��)=c(CH3COO��)��c(OH��)��c(H��)����C����
D�� ͼ��a����Һ�к��е�Ũ�ȵĴ����ƺ��������ƣ����������غ㣺c(Na��)= 2c(CH3COO��)��2c(CH3COOH)�����ݵ���غ�c(Na��)+ c(H��)=c(CH3COO��) +c(OH��)��������ʽ�ɵã�c(OH��)��c(H��)��c(CH3COO��)��2c(CH3COOH)����D��ȷ��
��ѡD��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������顱���ºϳɵ�һ����������ӳ�������ṹ���÷��ӵ�̼�ܽṹ����ͼ��ʾ������ÿ�������ʾһ��̼ԭ�ӣ����ʾ̼̼��������ԭ��δ������
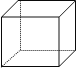
��1���������顱�ķ���ʽΪ___________��
��2����һ�ȴ��ﹲ��_______�֣�
��3������ȴ��ﹲ��_______�֡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ�������£�ij�����и����ڷ�Ӧǰ��仯��ʾ��ͼ���£�����![]() ��
��![]() ������ͬԪ�ص�ԭ�ӡ����ڴ˷�Ӧ˵������ȷ����
������ͬԪ�ص�ԭ�ӡ����ڴ˷�Ӧ˵������ȷ����
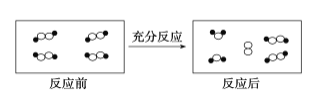
A.���������е���B.��Ӧ��ȫ�����뷴Ӧ
C.����������ԭ��ӦD.���ڷֽⷴӦ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����NO�Ʊ�NH4NO3�Ĺ���ԭ����ͼ��ʾ��X��Y��Pt�缫��Ϊʹ������ȫ��ת��ΪNH4NO3���貹������A������˵����ȷ���ǣ� ��
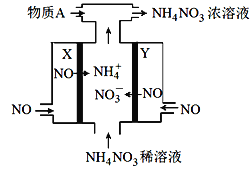
A. ����AΪNH3
B. X�缫Ϊ��������
C. Y�缫�Ϸ����˻�ԭ��Ӧ
D. Y�缫��Ӧʽ��NO��3e��+ 4OH��===NO3- +2H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�������ͼΪ����ʵ��װ�á�
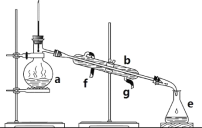
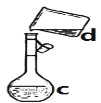
��1��д���������������ƣ�a.____________��b.___________��
��2��������װ�÷������Ȼ�̼�;ƾ��Ļ�����ȱ�ٵ�������__________���������������������ʵ�飬�¶ȼ�ˮ�����λ����______����
��.����һ�����ʵ���Ũ�ȵ���Һ�ǻ�ѧʵ���ҵĻ���ʵ�����֮һ����ش��������⣺
��3������0.5 mol/L��������Һ450 mL��������Ͳ��ȡ��������98%���ܶ�1.84 g/cm3��Ũ��������Ϊ_____mL�����ʵ������15 mL��20 mL��50 mL ��Ͳ��Ӧ���ѡ��_____mL��Ͳ��
��4������������Һ�����õ���Ͳ���ձ����������⣬����Ҫ�����ֲ���������_____��_______��
��5������ʱ������ȷ�IJ���˳����__________������ĸ��ʾ��ÿ������ֻ��һ�Σ���
A��������ˮϴ���ձ�2�Ρ�3�Σ�ϴ��Һ��ע������ƿ����
B����ʢ��ˮ���ձ��м���Ũ����ϡ��
C�����ձ�������ȴ����Һ�ز�����ע������ƿ��
D��������ƿ�ǽ����������µߵ���ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ��Һ��ǡ����̶�����
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1 cm��2 cm��
��6�������������������������ҺŨ�Ƚ��к�Ӱ�죨�ƫ�ߡ�����ƫ�͡�����Ӱ�족������û�н���A����___________��������ʱ���ӿ̶���_______
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͭ��ijɷ���Cu2O����ͭ��ijɷ���Cu2S������ͭ�����ͭ���ϼ��������·�Ӧ��2Cu2O��Cu2S![]() 6Cu��SO2�������ڸ÷�Ӧ������˵����ȷ����
6Cu��SO2�������ڸ÷�Ӧ������˵����ȷ����
A. �÷�Ӧ��������ֻ��Cu2O B. Cu��������������ǻ�ԭ����
C. Cu2S�������������ǻ�ԭ�� D. ��ԭ������������������ʵ���֮��Ϊ1��6
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������йص绯ѧװ�õ�˵����ȷ����
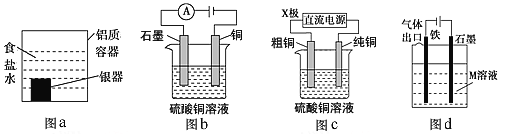
A. ����ͼaװ�ô�����������ĺڰ�Ag2S����������ķ�ӦΪAg2S��2e��=2Ag��S2��
B. ͼb���һ��ʱ�䣬ͭ�缫�ܽ⣬ʯī�缫��������ɫ��������
C. ͼc�е�X����Ϊ���������װ�ÿ�ʵ�ִ�ͭ�ľ���
D. ͼd����M�Ǻ�ˮ����װ����ͨ��������������������������ʹ��������ʴ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ��ȤС���ͬѧ����ͼ��ʾװ���о��йص绯ѧ�����⡣���պϸ�װ �õĿ���ʱ���۲쵽��������ָ�뷢����ƫת��

��ش��������⣺
��1���׳�Ϊ_____(����ԭ�������������������Ƴ���)��ͨ�� CH3OH �缫�ĵ缫��ӦΪ_____��
��2���ҳ��� A(ʯī)�缫������Ϊ_____(������������������������������������)���ܷ�ӦΪ__________��
��3�����ס��ҡ�����Һ�����Ϊ500 mL�����ҳ��� B ���������� 5.4 g ʱ���׳������������� O2 �����Ϊ_____mL(��״ ��)���ҳ�����ҺPH=_______��������_____(����C������D��)������_____g ͭ��
��4�������е缫���䣬������Һ���� NaCl ��Һ�����رպ�һ��ʱ�������Һ�� pH��_____(��������������С����������������ͬ)��������Һ�� pH ��______��
��5��ij��Һ�п��ܺ������������е����ֻ��֣�Ba2+��H+��SO42-�� SO32- ��HCO3-�� Cl-��
�ٵ���Һ���д���H+����ʱ��������_______________________���Ӵ��ڡ�
�ڵ���Һ���д���Ba2+����ʱ����Һ�в�������_________________���Ӵ��ڡ�
�۲��ö��Ե缫������������ѡ���ʵ��������������ˮ�ĵ���ʣ�������Һ���е���������ֱ�ų����壬�������Ϊ1��1�������ʻ�ѧʽ��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��д���������ʵĵ��뷽��ʽ:
��H2SO4: ________
��NaOH: ________
��Fe2��SO4��3: ________
��2��д�����л�ѧ��Ӧ�����ӷ���ʽ:
���������������ᷴӦ��________
����Ƭ������ķ�Ӧ��________
������ͭ�����������ķ�Ӧ��________
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com