
(18��)������I(C11H12O3)���Ʊ�Һ�����ϵ��м���֮һ��������к���ȩ����������I������E��H��һ�������ºϳɣ�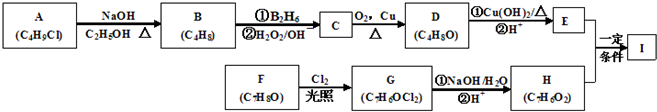
��֪������Ϣ���� A�ĺ˴Ź������ױ�����ֻ��һ�ֻ�ѧ�������⣻
��R��CH��CH2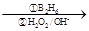 R��CH2CH2OH���ۻ�����F�����ϵ�һ�ȴ���ֻ�����֣���ͨ����ͬһ��̼ԭ�������������ǻ����ȶ�������ˮ�γ��ʻ���
R��CH2CH2OH���ۻ�����F�����ϵ�һ�ȴ���ֻ�����֣���ͨ����ͬһ��̼ԭ�������������ǻ����ȶ�������ˮ�γ��ʻ���
��ش��������⣺
(1)A�Ľṹ��ʽΪ ��B���������ŵ������� ��
(2)C������Ϊ ��E�ķ���ʽΪ ��
(3)A��B��C��D��F��G�ķ�Ӧ���ͷֱ�Ϊ �� �� ��
(4)д�����з�Ӧ�Ļ�ѧ����ʽ��
D��E�ڢٲ���Ӧ ��
F��G ��
(5)I�ĽṹͲʽΪ ��
(6)I��ͬϵ��J��I��Է�������С14��J��ͬ���칹������ͬʱ���������������ٱ�����ֻ������ȡ�������ڼ��ܷ���������Ӧ�������뱥��NaHCO3��Һ��Ӧ�ų�CO2������ �֣������������칹)��J��һ��ͬ���칹�巢��������Ӧ���ữ��˴Ź�������Ϊ����壬�ҷ������Ϊ2��2��1��д��J������ͬ���칹��Ľṹ��ʽ ��
��1��(CH3)3CCl��̼̼˫�� ��2��2������1��������C4H8O2
��3����ȥ��Ӧ��������Ӧ��ȡ����Ӧ
��4��(CH3)2CHCHO��2Cu(OH)2��NaOH (CH3)2CHCOONa��3H2O��Cu2O��
(CH3)2CHCOONa��3H2O��Cu2O��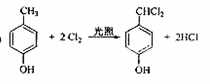 ��5��
��5��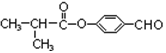
��6��18��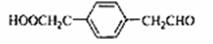 ��
��
�������������A�ķ���ʽΪC4H9Cl���˴Ź������ױ�����ֻ��һ�ֻ�ѧ�������⣬����A�Ľṹ��ʽΪ(CH3)3CCl��A��B����ȥ��Ӧ��B��(CH3)2C��CH2��B��C�Ǽӳɷ�Ӧ�������ṩ�ķ�Ӧ��Ϣ��C��(CH3)2CHCH2OH��C��D��������Ӧ��D��(CH3)2CHCHO��D��E��������Ӧ��E��(CH3)2CHCOOH��F�ķ���ʽΪC7H8O�������ϵ�һ�ȴ���ֻ�����֣�����F�Ľṹ��ʽΪ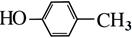 ���ڹ��������������������ʵ���֮��1:2��Ӧ���ǶԼ��еļ��е�2��H��Clȡ�������G�Ľṹ��ʽΪ
���ڹ��������������������ʵ���֮��1:2��Ӧ���ǶԼ��еļ��е�2��H��Clȡ�������G�Ľṹ��ʽΪ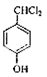 ��G��H��ˮ�ⷴӦ��������Ϣ��Ӧ���ǡ�CHCl2��ɡ�CHO����H�Ľṹ��ʽΪ
��G��H��ˮ�ⷴӦ��������Ϣ��Ӧ���ǡ�CHCl2��ɡ�CHO����H�Ľṹ��ʽΪ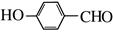 ��H��I��������Ӧ��I�Ľṹ��ʽΪ��
��H��I��������Ӧ��I�Ľṹ��ʽΪ��  ��
��
��1���������Ϸ�����֪��A�Ľṹ��ʽΪ(CH3)3CCl��B���������ŵ�������̼̼˫����
��2��C������Ϊ2������1��������E�ķ���ʽΪC4H8O2��
��3��A��B��C��D��F��G�ķ�Ӧ���ͷֱ�Ϊ��ȥ��Ӧ��������Ӧ��ȡ����Ӧ��
��4��ȩ��������������ͭ����Һ��Ӧ�Ļ�ѧ����ʽΪ(CH3)2CHCHO��2Cu(OH)2��NaOH (CH3)2CHCOONa��3H2O��Cu2O���������ڹ�������������������������ԭ�ӵ�ȡ����Ӧ����Ӧ�Ļ�ѧ����ʽΪ
(CH3)2CHCOONa��3H2O��Cu2O���������ڹ�������������������������ԭ�ӵ�ȡ����Ӧ����Ӧ�Ļ�ѧ����ʽΪ ��
��
��5��I�Ľṹ��ʽΪ��  ��
��
��6��J��I��ͬϵ���Է�������С14��˵��J��I��һ��Cԭ�ӣ�����ȡ���������ܷ���������Ӧ�����ܺͱ���NaHCO3��Һ��Ӧ�ų�CO2��������һ�����Ȼ���һ��ȩ������COOH�롪CH2CH2CHO��ϣ���COOH�롪CH(CH3)CHO��ϣ���CH2COOH�롪CH2CHO��ϣ���CH2CH2COOH�롪CHO��ϣ���CH(CH3)COOH�롪CHO��ϣ���HOOCCH��CHO�����롪CH3����ϣ�ÿһ����Ͽ����ڡ��䡢������λ�ñ仯��һ����6��3=18������������ͬ���칹�塣���У�һ��ͬ���칹�巢��������Ӧ���ữ��˴Ź�������Ϊ����壬�ҷ������Ϊ2:2:1���ṹ��ʽΪ�� ��
��
���㣺�����л�������֪ʶ���漰�л��������л��ṹ��ʽ������ʽ����ѧ����ʽ��ͬ���칹�������ж�


 �����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д�
�����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д� �����ҵ�����������ѧ���ӳ�����ϵ�д�
�����ҵ�����������ѧ���ӳ�����ϵ�д� ����ѧ��Ӯ�����ϵ�д�
����ѧ��Ӯ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��14�֣��ҹ����ϡ������ȵ�ʢ����ɽ������������ȩ�����ܸߣ����������ɴﵽ60%��90%������ȩҲ�������������ϩΪԭ���˹��ϳɣ�����ȩ�ֿ������ϳ�������ͪ���㾫���ϣ���ϳ�·�����£�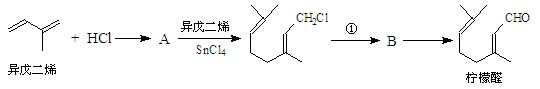

��ͬһ̼ԭ����������˫���ṹ���ȶ���
�Ը�������ת����ϵ�ش��������⣺
��1��д��A�Ľṹ��ʽ ��C�Ľṹ��ʽ ��
��2���ٶ�Ӧ�ķ�Ӧ������ ����Ӧ�۵ķ�Ӧ������ ��
��3��д��Bת��Ϊ����ȩ�Ļ�ѧ����ʽ ��
��4�����ݷ�Ӧ�ڵķ�Ӧ����д��CH3CHO��������HCHO��Ӧ����Ľṹ��ʽ��
��
��5����������ȩ�к���̼̼˫����ʵ�鷽���ǣ� ��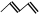
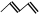 ��6������������ͪ���£�������ͪ�кܶ�ͬ���칹�壬����������������ͬ���칹���� �֡�
��6������������ͪ���£�������ͪ�кܶ�ͬ���칹�壬����������������ͬ���칹���� �֡�
�ٺ���һ������ �����ڴ����Ҳ��ܷ�����������Ӧ
�ۺ˴Ź���������ʾ��5����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(14)��֪����˫�������˵�ϩ���������⻯һ������Ӧ�����ɵĴ��ǻ������ˣ�
R-CH=CH2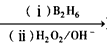 R-CH2CH2OH
R-CH2CH2OH
��RCH2CHO +R'CH2CHO 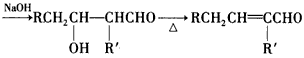
������M��һ�����ϣ���������·�ߺϳ�
��֪���˴Ź���������ʾE��������������ԭ�ӣ�FΪ���㻯�����ش��������⣺
��1�� д����ӦA��C�ķ�Ӧ������___________ __________��
��2��. ԭ��C4H10��������_________ _____��ϵͳ��������
��3�� д��M�Ľṹ��ʽ��________ ___��
��4�� д��E��N����F�Ļ�ѧ����ʽ��_____________________ _____��
��5�� д��D��G����M�Ļ�ѧ����ʽ��_______ ______________ ____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
[��ѧһѡ��2����ѧ�뼼��]��15�֣�
ˮ��һ����Ҫ����Ȼ��Դ���������������治��ȱ�ٵ����ʡ�ˮ�Ĵ�����Ҫ����ˮ�ľ�
������ˮ������Ӳˮ�����ͺ�ˮ�ĵ����ȡ�
��1����ˮ�ĵ������õķ�����_________��_________�����������ȡ�
��2�����������ǽ�������չ������һ�ֽϺõĺ�ˮ������������ԭ����ͼ��ʾ��
�����������Ե缫���ĵ缫��ӦʽΪ __________________.
�ڵ�ˮ�ij���Ϊa��b��c�е�_________ ���ڡ�
��ij�����ų���Ũ��ˮ����Ԫ�صĺ���Ϊ0.68g/L���������������е�����������Ϊ�嵥�ʣ�����l.0��10 L��Ũ��ˮ�����µ����������Ϊ_________
L��Ũ��ˮ�����µ����������Ϊ_________ ��
��
��3�� K2FeO4������ˮ�����õ�����������������ҵ�ϳ���Fe(NO3)3��Һ��ŨKCIO��Һ��ǿ���Ի���������K2FeO4���÷�Ӧ�����ӷ���ʽΪ___________________________.
��4���ȼҵ����_________������������������ӽ���Ĥ���۵�⾫�Ƶı���ʳ��ˮ�� ��ʳ��ˮ�к�������MgCl2�������ӽ���Ĥ�����������ԭ����_______ (�����ӷ���ʽ��ʾ����
��5����������ABS�ϳ�ʱ�������������ֵ��壺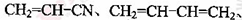 ��
�� ����д��ABS�Ľṹ��ʽ_______________________________��
����д��ABS�Ľṹ��ʽ_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ij������A����������£�����ͼ��ʾ������ʺɱ�Ϊ120�����������ʾ�������������˴Ź���������ʾ�����ַ����������Ϊ1:2:2:1:6��A���л��ϳ����ܷ�������ת����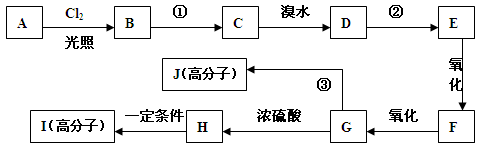
����֪����������ȡ����Ӧ�Ļ��Դ���Ϊ CH > CH2 > CH3 ������>����>���⣩
�ش��������⣺
��1��д��A�Ľṹ��ʽ ��
��2�� ��Ӧ�ٺ͢���ͬ�ķ�Ӧ�Լ�������Ϊ ��
��3��F�����еĺ��������������� ��
��4����Ӧ�۵Ļ�ѧ����ʽΪ�� ��
��5��д����������������H���ӵ�����ͬ���칹��Ľṹ��ʽ��
�ٺ��б��� ��1 mol�л����������Ӧ����4 mol Ag �۱����ϵ�һ�����������
��
��6����H������ͬ�����ŵ�H��ͬ���칹�������֣�����һ�ַ���K������˳���칹
��,��д��K�Ľṹ��ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ۻ�ѧ�D�Dѡ��5���л���ѧ�����ݣ�15�֣�
�����ܻ����ɸ��ưƵ����ϵĿڸУ�����Ӧ�����涨��������DBP��һ�������ܻ�������������·�ߺϳɣ�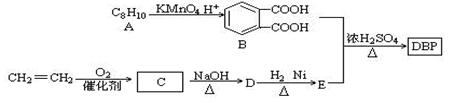
��֪������Ϣ��
��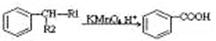
��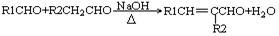 ����R1����R2��ʾ��ԭ�ӻ�������
����R1����R2��ʾ��ԭ�ӻ�������
��1��A�Ľṹ��ʽ������������D�Ľṹ��ʽ����������������D��E�ķ�Ӧ������������������
��2��D��H2 1�U1��Ӧ����E����E����������Ϊ_________��DBP�ķ���ʽΪ��������
��3����B��E�����ʵ�����1�U2�ϳ�DBP�Ļ�ѧ����ʽ�������������������� ��
��4��д��2��ͬʱ��������������B��ͬ���칹��ṹ��ʽ�������������� ��
���ܺ�NaHCO3��Һ��Ӧ����CO2��������ʹFeC13��Һ������ɫ��Ӧ
���ܷ���������Ӧ���������������� �ܱ����Ϻ�̼���Ŵ��ڶ�λ
��5��д��B��̼��������Һ��Ӧ�ķ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(15��)д�����з�Ӧ�Ļ�ѧ����ʽ��
��1����ȩ��������Һ��Ӧ ��
��2��������Һ��Ũ��ˮ�ķ�Ӧ ��
��3��1-������NaOH����Һ���� ��
��4���ñ����屽�ķ�Ӧ ��
��5���Ҵ��Ĵ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������һ�������˿ڵĽ�����衣���������ijɷ��ж��֣����ᱽ����
( )�����е�һ�֣������Դ���������ȡ��Ҳ�����üױ����Ҵ�Ϊԭ�Ͻ����˹��ϳɡ�һ�ֺϳ�·�����£�
)�����е�һ�֣������Դ���������ȡ��Ҳ�����üױ����Ҵ�Ϊԭ�Ͻ����˹��ϳɡ�һ�ֺϳ�·�����£�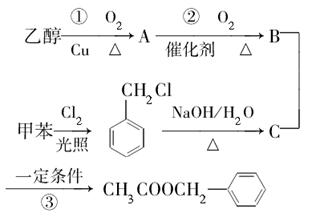
(1)C�Ľṹ��ʽΪ ��
(2)д����Ӧ�ٵĻ�ѧ����ʽ�� ��
(3)��Ӧ�۵ķ�Ӧ����Ϊ ��
(4)��Ӧ (�����)ԭ�ӵ�����������Ϊ100%��������ɫ��ѧ��Ҫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������һ������ƫͷʹ����Ч��ҩ��������ij�о�С�鿪�������������صĺϳ�·�ߡ�
��֪:��A����Է�������Ϊ104,1 mol A��������̼�����Ʒ�Ӧ����44.8 L���壨��״����;
��B�Ľṹ�к���ȩ��;
��C��һ�������������л���D;
��RCHO+HOOC��CH2COOH RCH
RCH C��COOH��2+H2O��RCH
C��COOH��2+H2O��RCH C��COOH��2
C��COOH��2 RCH
RCH CHCOOH+CO2����
CHCOOH+CO2����
��ش��������⡣
��1��A�ķ���ʽ����������,B�Ľṹ��ʽΪ����������
��2��C���ܷ����ķ�Ӧ����������������ţ���
| A��������Ӧ | B��ˮ�ⷴӦ |
| C����ȥ��Ӧ | D��������Ӧ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com