
��֪��������ʱH2O H++OH-��KW=10-14�� CH3COOH
H++OH-��KW=10-14�� CH3COOH  H++
CH3COO����Ka=1.8��10-5
H++
CH3COO����Ka=1.8��10-5
��1��ȡ����������Һ���������������ƹ��壬��ʱ��Һ��C��H+����C��CH3COOH���ı�ֵ�� �� �������С�����䡱��
��2��������ˮ������ӷ���ʽΪ�������� �������� ���������¶�ʱ��C(OH��)�������������������С�������䡱����
��3��0��5mol��L-1��������ҺpHΪm����ˮ��ij̶ȣ���ˮ��Ĵ�������ԭ�д����Ƶı�ֵ��Ϊa��1mol��L-1��������ҺpHΪn��ˮ��ij̶�Ϊb����m��n�Ĺ�ϵΪ�������� ��a��b�Ĺ�ϵΪ����������������ڡ���С�ڡ������ڡ�����
��4�����������Ũ�ȵĴ��������������Һ��Ϻ�������Һ������Ũ���ɴ�С��˳�������������������� ������ ��
��5�������������������Һ��Ϻ�pH<7����c��Na+��_______________ c��CH3COO����������ڡ�����С�ڡ����ڡ�����
��6������ʱ������pH��3��HA��ҺV1mL��pH��11��NaOH��ҺV2 mL��ϣ�������˵������ȷ����____________��
A������Ӧ����Һ�����ԣ���c��H+��+c��OH������2��10��7mol��L��1
B����V1=V2����Ӧ����ҺpHһ������7
C������Ӧ����Һ�����ԣ���V1һ������V2
D������Ӧ����Һ�ʼ��ԣ���V1һ��С��V2
��7����ij��Һ�к�Mg2+��Cd2+��Zn2+�������ӵ�Ũ�Ⱦ�Ϊ0.01mol��L-1�������м����
������ƺ�����Һ��C(OH-)Ϊ2.2��10-5mol��L-1���������ֽ��������� �����ɳ�����ԭ�������� ������������������������������������������������������ ����
��KSP��Mg��OH��2��=1.8��10-11��KSP��Zn��OH��2��=1.2��10-17��
KSP��Cd��OH��2��=2.5��10-14��
��8��ȡ10mL0.5mol��L-1������Һ����ˮϡ�͵�500mL�������Һ����ˮ�������c��H+��
=������ ��mol/L��
��1������2��CH3COO��
+ H2O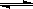 CH3COOH+OH-������3��С�ڣ����ڣ�4��c��Na+����c��CH3COO-����c��OH-����c��H+����5��С�ڣ�6��BC��7��Cd2+��Zn2+����OH-��=2.2��10-5mol��L-1
��M2+�ݣ�OH-��2=5��10-12��mol��L-1��35��10-12С��KSP��Mg��OH��2��=1.8��10-115��10-12����KSP��Zn��OH��2��=1.2��10-175��10-12����KSP��Cd��OH��2��=2.5��10-14��8��1��10-12
CH3COOH+OH-������3��С�ڣ����ڣ�4��c��Na+����c��CH3COO-����c��OH-����c��H+����5��С�ڣ�6��BC��7��Cd2+��Zn2+����OH-��=2.2��10-5mol��L-1
��M2+�ݣ�OH-��2=5��10-12��mol��L-1��35��10-12С��KSP��Mg��OH��2��=1.8��10-115��10-12����KSP��Zn��OH��2��=1.2��10-175��10-12����KSP��Cd��OH��2��=2.5��10-14��8��1��10-12
��������
�����������1��������ƹ���������CH3COO-��c��CH3COO-������ʹ��ƽ��CH3COOH⇌CH3COO-+H+�����ƶ���C��H+����С��C��CH3COOH����������C��H+����C��CH3COOH���ı�ֵ��С���ʴ�Ϊ����С����2��������ˮ������ӷ���ʽΪ��CH3COO-+H2O⇌CH3COOH+OH-���÷�Ӧ�����ȷ�Ӧ���¶����ߣ���Һ������������Ũ�����ʴ�Ϊ��CH3COO-+H2O⇌CH3COOH+OH-������3�����ڴ�������Һ�У������Ƶ�Ũ��Խ��ˮ��̶�ԽС��������Һ�е����������ӵ����ʵ���Ũ�ȷ���Խ����Һ��PHԽ������mС��n��a����b���ʴ�Ϊ��С�ڣ����ڣ���4������Ϊ������ʣ���������������ʵ���Ũ�ȵĴ��������������Һ��Ϻ�ǡ������CH3COONa��Ϊǿ�������Σ���Һ�ʼ��ԣ�c��OH-����c��H+������Һ�д��ڣ�c��CH3COO-��+c��OH-��=c��Na+��+c��H+������c��Na+����c��CH3COO-�����ʴ�Ϊ��c��Na+����c��CH3COO-����c��OH-����c��H+������5��������Һ��PHС��7����Һ�����������ʵ���Ũ�ȴ������������ӵ�Ũ�ȣ���������Ҫ�Ǵ������ģ����Դ��������Ũ�ȴ���������Ũ�ȣ��ʴ�Ϊ��С�ڣ���6��A������Ӧ����Һ�����ԣ���Һ��������Ũ��=����������Ũ��=1��10-7mol•L-1c��H+��+c��OH-��=2��10-7mol•L-1����A˵����ȷ��B��������������ʲ��ֵ��룬�����Ũ�ȴ����������Ƶ�Ũ�ȣ���V1=V2����Ӧ����ҺpHһ��С��7����B˵������C������Ӧ����Һ�����ԣ����ڴ���Ũ�ȴ�����������Ũ�ȣ���V1��V2����C˵������D������Ӧ����Һ�ʼ��ԣ�����V1=V2��Һ��ʾ���ԣ�����V1һ��С��V2����D˵����ȷ����7����Һ������������Ũ���ǣ�[OH-]=2.2��10-5mol•L-1������[M2+][OH-]2=5��10-12��mol•L-1��3������5��10-12С��KSP[M��OH��2]=1.8��10-11��û��������þ�������ɣ�����5��10-12����KSP[Zn��OH��2]=1.2��10-17����������п�����ɣ�����5��10-12����KSP[Cd��OH��2]=2.5��10-14����Cd��OH��2�������ɣ��ʴ�Ϊ��Cd2+��Zn2+����8��ϡ�ͺ���Һ�е�������Ũ���ǣ�c��H+��=0.5mol•L−1��0.01L/0.5L=0.01mol/L������������Һ�У�ˮ�����������Ũ�ȵ�����Һ�е����������ӵ�Ũ�ȣ�����Һ����ˮ�������c��H+��=1��10-12���ʴ�Ϊ��1��10-12��
���㣺���������ˮ��Һ�еĵ���ƽ�⣻���ܵ���ʵ��ܽ�ƽ�⼰����ת���ı��ʣ������ʱ�Ķ����жϼ��й�pH�ļ��㡣


 ����ʦ���һ��һ��ϵ�д�
����ʦ���һ��һ��ϵ�д� �Ͻ�ƽ��У����ϵ�д�
�Ͻ�ƽ��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| 36cV��10-3 |
| a |
| 36cV��10-3 |
| a |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�걱�������ƶ��и߶����£����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com