
�±���25��ʱijЩ�ε��ܶȻ�����������ĵ���ƽ�ⳣ��������˵����ȷ����
|
��ѧʽ |
AgCl |
Ag2CrO4 |
CH3COOH |
HClO |
H2CO3 |
|
Ks�� Ka |
Ksp=1.8��10-10 |
Ksp=2��10-12 |
Ka=1.8��10-5 |
Ka=3.0��10-8 |
Ka1=4.1��10-7 Ka2=5.6��10-11 |
A����ͬŨ��CH3COONa��NaClO�Ļ��Һ�У�������Ũ�ȵĴ�С��ϵ��c��Na+��>c��ClO-��>c��CH3COO-��>c��OH-��>c��H+��
B����0.1mol��L-1CH3COOH��Һ�еμ�NaOH��Һ��c(CH3COOH)��c(CH3COO-)=5 ��9����ʱ��ҺpH=5
C��̼������Һ�еμ�������ˮ�����ӷ���ʽΪCO32-+Cl2=HCO-3+Cl-+HClO
D����Ũ�Ⱦ�Ϊ1��10-3mol��L-1��KCl��K2CrO4���Һ�еμ�1��10-3mol��L-1��AgNO3��Һ��CrO42-���γɳ���
B
������������ĵ��볣�����ڴ�����ģ����Դ������Ƶ�ˮ��̶ȴ��ڴ����Ƶģ�A����ȷ��Ӧ����c��Na+��>c��CH3COO-��>c��ClO-��>c��OH-��>c��H+����B����������Ũ��Ϊ5x�����������ӵ�Ũ��Ϊ9x�����ݵ��볣������ʽ��֪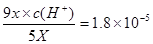 �����������Ũ����10��5mol/L������pH��5��B��ȷ��C��̼���ƹ�������������ĵ��볣������̼��ĵڶ������룬���Դ�������Ժ�̼���Ʒ�Ӧ���ɴ������ƺ�̼�����ƣ�C����ȷ�������ܶȻ�������֪ʹ�Ȼ���������������Ũ����1.8��10-7mol��L-1����ʹ������������������Ũ�ȴ���1.8��10-7mol��L-1������Ȼ����ȳ���������ѡ��D����ȷ����ѡB��
�����������Ũ����10��5mol/L������pH��5��B��ȷ��C��̼���ƹ�������������ĵ��볣������̼��ĵڶ������룬���Դ�������Ժ�̼���Ʒ�Ӧ���ɴ������ƺ�̼�����ƣ�C����ȷ�������ܶȻ�������֪ʹ�Ȼ���������������Ũ����1.8��10-7mol��L-1����ʹ������������������Ũ�ȴ���1.8��10-7mol��L-1������Ȼ����ȳ���������ѡ��D����ȷ����ѡB��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2012?�㽭ģ�⣩�±���25��CʱijЩ�ε�Ũ�Ȼ�����������ĵ���ƽ�ⳣ��������˵����ȷ���ǣ�������
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�±���25��ʱijЩ�ε��ܶȻ�����������ĵ���ƽ�ⳣ��������˵����ȷ����
| ��ѧʽ | AgCl | Ag2CrO4 | CH3COOH | HClO | H2CO3 |
| Ks�� Ka | Ksp=1.8��10-10 | Ksp=2��10-12 | Ka=1.8��10-5 | Ka=3.0��10-8 | Ka1=4.1��10-7 Ka2=5.6��10-11 |
A����ͬŨ��CH3COONa��NaClO�Ļ��Һ�У�������Ũ�ȵĴ�С��ϵ��c��Na+��>c��ClO-��>c��CH3COO-��>c��OH-��>c��H+��
B����0.1mol��L-1CH3COOH��Һ�еμ�NaOH��Һ��c(CH3COOH)��c(CH3COO-)=5 ��9����ʱ��ҺpH=5
C��̼������Һ�еμ�������ˮ�����ӷ���ʽΪCO32-+Cl2=HCO-3+Cl-+HClO
D����Ũ�Ⱦ�Ϊ1��10-3mol��L-1��KCl��K2CrO4���Һ�еμ�1��10-3mol��L-1��AgNO3��Һ��CrO42-���γɳ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ�������и�����һ�����Ͽ��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�±���25��CʱijЩ�ε�Ũ�Ȼ�����������ĵ���ƽ�ⳣ��������˵����ȷ���ǣ� ��
|
��ѧʽ |
AgCl |
Ag2CrO4 |
CH3COOH |
HClO |
H2CO3 |
|
KSP��Ka |
KSP=1.8��10-10 |
KSP=2.0��10-12 |
Ka=1.8��10-5 |
Ka=3.0��10-8 |
Ka1=4.1��10-7 Ka2=5.6��10-11 |
A����ͬŨ��CH3COONa��NaClO�Ļ��Һ�У�������Ũ�ȵĴ�С��ϵ��
c��Na+��>c��ClO-��>c��CH3COO-��>c��OH-��>c��H+��
B��̼������Һ�еμ�������ˮ�����ӷ���ʽΪCO32-+Cl2=HCO3-+Cl-+HClO
C����0.1mol��L-1CH3COOH��Һ�еμ�NaOH��Һ��c��CH3COOH����c��CH3COO-��=5��9����ʱ��ҺpH=5
D����Ũ�Ⱦ�Ϊ1��10-3mol��L-1��KCl��K2CrO4���Һ�еμ�1��10-3mol��L-1��AgNO3��Һ��CrO42-���γɳ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ�����в�����ѧ�߶�5���¿���ѧ�Ծ� ���������� ���ͣ���ѡ��
�±���25��ʱijЩ�ε��ܶȻ�����������ĵ���ƽ�ⳣ��������˵����ȷ����
| ��ѧʽ | AgCl | Ag2CrO4 | CH3COOH | HClO | H2CO3 |
| Ks�� Ka | Ksp=1.8��10-10 | Ksp=2��10-12 | Ka=1.8��10-5 | Ka=3.0��10-8 | Ka1=4.1��10-7 Ka2=5.6��10-11 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com