
��ѧ�ڻ��������Խ��Խ��ʾ����Ҫ���ã�ˮ���ܻ�ԭ�����ʣ���Ҫ���л����Ⱦ�ij̶ȳ��û�ѧ��������COD����������COD��ָ��һ�������£���ǿ����������һ�����ˮ��ʱ�����ĵ�ǿ�������൱����������������λ��mg/L��ʾ��
�ظ���ط���ָ��ǿ������Һ�У���һ�������ظ������������Ag+��������ˮ���еĻ�ԭ�����ʣ��䱾������ԭΪCr3+���������ظ����������������ָʾ�����������������Һ�صΣ���������Լ��������������ˮ���Ļ�ѧ��������COD����
��ش��������⣺
��1����ˮ���к���Ũ�Ƚϸߵ�Cl��ʱ���������HgSO4��Һ����HgSO4�����ü�����ԭ���� ��
��2���ζ�ʱ�����������Һ��ʢ���� ����ᡱ���ʽ�ζ����С�
��3�����ҡ���������ˮ�����鷨��GB5750-85�����з���ļ������Ϊ0.002 mg��L��ijˮԴ�ܻ�������Ⱦ���Ӻ�����0.282 mg/L������Ⱦ��ˮ���зӣ��Ա���������CO2���㣩����Ļ�ѧ��������COD��Ϊ mg/L��
��4��ij��������������ҵ�ŷų��ķ�ˮ�У�ij��COD����ֵ�ߴ�2100 mg/L����������ҵ���п����� ��������ĸ��
a�����dz� b��ˮ�೧ c����Ƴ� d����ֽ��
��5������ij��ˮ��Ʒ10.00 mL���ȼ���10 mL����ˮ��Ȼ�����10.00 mL 0.04000 mol/L K2Cr2O7��Һ��3 mL 1%����-��������Һ��17 mL���ṯ��Һ�����ȷ�Ӧ2 h������������ָʾ����������0.1000 mol/L Fe(NH4)2(SO4)2��Һ�ζ������K2Cr2O7������ȥFe(NH4)2(SO4)2��Һ12.00 mL���Լ����ˮ���Ļ�ѧ��������COD����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ�ڻ��������г���ʮ����Ҫ�����ã������������͵绯ѧ���ⷨ����������ˮ�������ε���Ⱦ��
(1) �����������У�H2�ܽ�NO3����ԭΪN2��25��ʱ����Ӧ����10min����Һ��pH��7��Ϊ12��
��N2�ĽṹʽΪ ��
��������Ӧ���ӷ���ʽΪ ��
��ƽ����Ӧ����v(NO3��)Ϊ mol ∙L��1 ∙min��1
�ۻ�ԭ�����п������м����NO2����д��3
�ִٽ�NO2��ˮ��ķ��� ��
(2)�绯ѧ����NO3����ԭ������11ͼ��ʾ��
�ٵ�Դ����Ϊ (�A����B��)��
������ӦʽΪ ��
������������ת����2mol���ӣ���Ĥ����
���Һ�������仯��(��m������m��)Ϊ g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ȫ����ͨ�ߵ�ѧУ����ͳһ���������ۺ��������Ի�ѧ��������������� ���ͣ������
��ѧ�ڻ�������������ʮ����Ҫ�����ã������������͵绯ѧ���ⷨ����������ˮ�������ε���Ⱦ��
��1�������������У�H2�ܽ�NO3����ԭΪN2��25��ʱ����Ӧ����10min����Һ��pH��7��Ϊ12��
��N2�ĽṹʽΪ ��
��������Ӧ�����ӷ���ʽΪ ����ƽ����Ӧ���ʦ�(NO3��)Ϊ mol��L��1min-1��
�ۻ�ԭ�����п������м����NO2����д��3�ִٽ�NO2��ˮ��ķ��� ��
��2���绯ѧ����NO3����ԭ����ͼ��ʾ��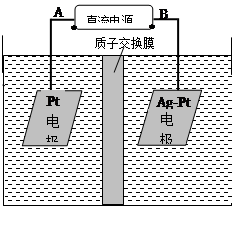
�ٵ�Դ����Ϊ ����A��B����������ӦʽΪ ��
������������ת����2mol���ӣ���Ĥ������Һ�������仯��(��m������m��)Ϊ g��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com