
 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A��Ũ���ḽ���������̣�NH3��HCl���巴Ӧ������NH4C1���� |
| B��Ũ���ḽ������������NH3��Ũ���������Ӧ |
| C���Ȼ�����Һ����ǣ�����Һһ����A1Cl3��Һ |
| D��ʪ��ĺ�ɫʯ����ֽ������NH3��ˮ��Һ�Լ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

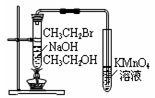
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����һ֧ʢ��2mL 2�� CuSO4��Һ���Թ��У����뼸��10����NaOH��Һ���ټ���1mL��ȩ��Һ�����Ⱥ���Կ�����ɫ������ͭ�������� |
| B��������������NaOH��Һ��ϲ���ˮԡ���ȣ����ɵõ�ˮ������Ҵ������� |
| C����������Ѿ�ˮ�⣬������������ϡ�������һ��ʱ�����NaOH��Һ�к����ᣬ�ټ���������Һ���� |
| D����һ֧�Թ��е���10�������飬�ټ���1mL 5����NaOH��Һ�����Ⱥ�μ���������Һ���ɹ۲쵽��dz��ɫ�廯���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ʽ�ζ���������ˮϴ����ֱ�Ӽ���δ֪Ũ�ȵ�NaOH��Һ |
| B����ƿ������ˮϴ����ֱ�Ӽ���һ�������δ֪Ũ�ȵ�NaOH��Һ |
| C���ζ�ǰ��û�������ʽ�ζ��ܼ��촦�����ݣ��ζ��յ�ʱ������ʧ |
| D���ζ�ǰ������ȷ���ﵽ�ζ��յ���Ӷ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | ʵ����� | ���� | ���ͻ���� |
| A | ������Fe���м���ϡHNO3����ַ�Ӧ����KSCN��Һ | ��Һ�ʺ�ɫ | ϡHNO3��Fe����ΪFe3�� |
| B | AgI�����е���ϡKCl��Һ | �а�ɫ�������� | AgCl��AgI������ |
| C | �����£���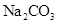 ��Һ�м����� ��Һ�м�����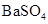 ��ĩ�����ˣ���ϴ���ij����м�ϡ���� ��ĩ�����ˣ���ϴ���ij����м�ϡ���� | �����ݲ��� | ����˵��������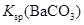 �� 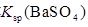 �Ĵ�С��ϵ �Ĵ�С��ϵ |
| D | �ò�����պȡŨ��ˮ�㵽��ɫʯ����ֽ�� | ��ֽ����ɫ | Ũ��ˮ�ʼ��� |
�鿴�𰸺ͽ���>>
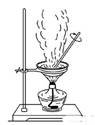
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
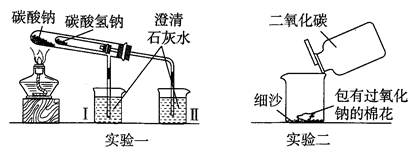
�鿴�𰸺ͽ���>>
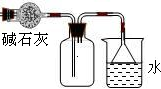
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
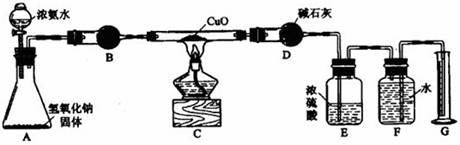
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ȡ����ˮʱ��Ϊ��ֹ��ƿ�ڲ�����������Ӧ��������ƿ�м��뼸Ƭ���Ƭ |
| B���ⶨδ֪NaOH��ҺŨ��ʱ����ʽ�ζ������ñ���Һ��ϴ2��3�� |
| C������0.1mol/L��H2SO4��Һʱ������ȡ��ŨH2SO4��������ƿ�м�ˮϡ�� |
| D��ȼ�ŵľƾ��Ʋ�������ʧ��Ӧ������ʪ������ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com