
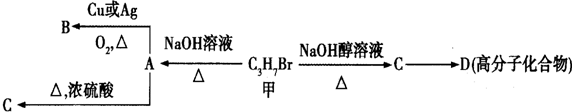
���� �ķ���ʽΪC3H7Br�����������ƵĴ���Һ�����������·�����ȥ��Ӧ����CΪCH3CH=CH2��C�����Ӿ۷�Ӧ����D����D�Ľṹ��ʽΪ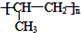 ��
��
��1�������������Ƶ�ˮ��Һ�����������·���ˮ�ⷴӦ����A��A��������B����B�ܷ���������Ӧ�����ΪCH3CH2CH2Br��AΪCH3CH2CH2OH��BΪCH3CH2CHO��
��2����B���ܷ���������Ӧ�����ΪCH3CHBrCH3��AΪCH3CH��OH��CH3��BΪ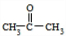 ��
��
��� �⣺�ķ���ʽΪC3H7Br�����������ƵĴ���Һ�����������·�����ȥ��Ӧ����CΪCH3CH=CH2��C�����Ӿ۷�Ӧ����D����D�Ľṹ��ʽΪ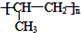 ��
��
��1�������������Ƶ�ˮ��Һ�����������·���ˮ�ⷴӦ����A��A��������B����B�ܷ���������Ӧ�����ΪCH3CH2CH2Br��AΪCH3CH2CH2OH��BΪCH3CH2CHO��B��������Һ��Ӧ�Ļ�ѧ����ʽ��CH3CH2CHO+2Ag��NH3��2OH$\stackrel{ˮԡ����}{��}$CH3CH2COONH4+3NH3+2Ag��+H2O��
�ʴ�Ϊ��CH3CH2CH2Br��CH3CH2CHO+2Ag��NH3��2OH$\stackrel{ˮԡ����}{��}$CH3CH2COONH4+3NH3+2Ag��+H2O��
��2����B���ܷ���������Ӧ�����ΪCH3CHBrCH3��AΪCH3CH��OH��CH3��BΪ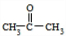 �������NaOH�Ĵ���Һ���ȷ�����Ӧ�Ļ�ѧ����ʽΪ��CH3CHBrCH3+NaOH$��_{��}^{��}$CH2=CHCH3��+NaBr+H2O��
�������NaOH�Ĵ���Һ���ȷ�����Ӧ�Ļ�ѧ����ʽΪ��CH3CHBrCH3+NaOH$��_{��}^{��}$CH2=CHCH3��+NaBr+H2O��
�ʴ�Ϊ��CH3CHBrCH3+NaOH$��_{��}^{��}$CH2=CHCH3��+NaBr+H2O��
���� ���⿼�����л�����ƶϣ��ؼ�����ȷC3H7Br��2��ͬ���칹�壬�ٽ�Ϸ�Ӧ���������ƶϣ����������л���Ĺ��������ʣ��ѶȲ���


 ��ڽ��ȫ������ϵ�д�
��ڽ��ȫ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ϵͳ������2��3-����-1��3-���ϩ��
��ϵͳ������2��3-����-1��3-���ϩ��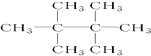 ��������2��2��3��3-�ļ����飻
��������2��2��3��3-�ļ����飻 ��
�� �Ľṹ��ʽCH2=CH��CH3��COOHCH3��
�Ľṹ��ʽCH2=CH��CH3��COOHCH3���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | X��Y��Z���ȶ������� | |
| B�� | A��B��C��Dֻ���γ�5�ֵ��� | |
| C�� | X��Y��Z���ֻ�������۷е������� | |
| D�� | ��A��B��C��D��Ԫ�ؼ��γɵĻ�������ֻ�����ۼ����������Ӽ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����Ԫ�ص�ԭ�Ӱ뾶�Ӵ�С��˳���ǣ�M��W��Z��Y��X | |
| B�� | X��Z��Ԫ�����γ�ԭ�Ӹ����ȣ�X��Z��Ϊ3��1�Ļ����� | |
| C�� | ������YW2��ZW2�������������� | |
| D�� | ��M������������ʯī�����������NaHCO3��Һ�����һ��ʱ���������������ְ�ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | -6 | B�� | +2 | C�� | +3 | D�� | +6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ɱ�����Ҫ�ɷ���H2O | B�� | ����CO�ɵ��������ж� | ||
| C�� | SO2�ɴ�������Ư��ʳƷ | D�� | С�մ����Ҫ�ɷ���Na2CO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ڱ�״���£�VLˮ���е���ԭ�Ӹ���Ϊ$\frac{V}{22.4}$NA | |
| B�� | CO2ͨ��Na2O2ʹ������bgʱ����Ӧ��ת�Ƶĵ�����ΪbNA/44�� | |
| C�� | �����£�1L 1 mol•L-1��������Һ�У�����CH3COO-ΪNA | |
| D�� | ���³�ѹ�£�0.1 mol�������еĵ�����ΪNA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Cl2 | B�� | Cl2O | C�� | Cl2O3 | D�� | ClO2 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com