
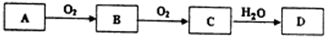
���� ��1����A��D��ˮ��Һ�������ԣ���DΪǿ�ᣬSԪ�ػ��������ת����ϵ������֪AΪH2S��BΪSO2��CΪSO3��DΪH2SO4��
��2����A��ˮ��Һ��ʹʪ��ĺ�ɫʯ����ֽ��������A��NH3��D��ϡ��Һ��ʹʪ�����ɫʯ����ֽ��죬Ϊ�ᣬ��������������NO��NO��������������NO2������B��NO��C��NO2��D��HNO3��
��� �⣺��1������A��D��ˮ��Һ�������ԣ���DΪǿ�ᣬSԪ�ػ��������ת����ϵ������֪��AΪH2S��BΪSO2��CΪSO3��DΪH2SO4���ʴ�Ϊ��H2S��H2SO4��
��B��Cת���Ļ�ѧ����ʽ��2SO2+O2$?_{��}^{����}$2SO3��
�ʴ�Ϊ��2SO2+O2$?_{��}^{����}$2SO3��
�ۼ�������SO2���ʵķ�������Bͨ�뵽Ʒ����Һ�У���Һ��ɫ�����Ⱥ��ָֻ�Ϊ��ɫ��
�ʴ�Ϊ����Bͨ�뵽Ʒ����Һ�У���Һ��ɫ�����Ⱥ��ָֻ�Ϊ��ɫ��
�������Ũ��Һ��Cu�ڼ��������Ļ�ѧ����ʽ��2H2SO4��Ũ��+Cu$\frac{\underline{\;\;��\;\;}}{\;}$CuSO4+2H2O+SO2����
�ʴ�Ϊ��2H2SO4��Ũ��+Cu$\frac{\underline{\;\;��\;\;}}{\;}$CuSO4+2H2O+SO2����
��2����A��ˮ��Һ��ʹʪ��ĺ�ɫʯ����ֽ��������A��NH3��D��ϡ��Һ��ʹʪ�����ɫʯ����ֽ��죬Ϊ�ᣬ��������������NO��NO��������������NO2������B��NO��C��NO2��D��HNO3��
�ٹ�ҵ�Ϻϳɰ����Ļ�ѧ����ʽ��N2+3H2$?_{����}^{���¸�ѹ}$2NH3����ʵ�����������Ȼ�����������Ƽ�����ȡ���就�����仯ѧ����ʽΪ��Ca��OH��2+2NH4Cl$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
�ʴ�Ϊ��N2+3H2$?_{����}^{���¸�ѹ}$2NH3��Ca��OH��2+2NH4Cl$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
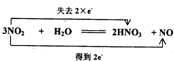
��C��D�Ļ�ѧ����ʽ������˫���ű������ת�Ƶķ������Ŀ�� ��
��
�ʴ�Ϊ�� ��
��
��A��B�Ļ�ѧ����ʽ��4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O��
�ʴ�Ϊ��4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O��
��Cu��Ũ���ᷴӦ��������ͭ�뵪�������������������������ˮ��Ӧ����ȫ��Ӧ�������ᣬ�ݹ��������̣�Cuʧȥ�������ʵ�������������õ������ʵ�����1.92gCu�����ʵ���Ϊ$\frac{1.92g}{64g/mol}$=0.03mol������Ҫ�������ʵ���Ϊ$\frac{0.03mol��2}{4}$=0.015mol������Ҫ�������Ϊ0.015mol��22.4L/mol=0.336L����Ϊ336mL��
�ʴ�Ϊ��336��
���� ���⿼�������ƶϣ��漰N��S��Ԫ�ص��ʼ��������ת�����Ѷ��еȣ�ּ�ڿ���ѧ����Ԫ�ػ�����֪ʶ�������գ�ע��������ѧ������������Ӧ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
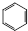 +HNO3$\stackrel{����}{��}$
+HNO3$\stackrel{����}{��}$ +H2O����Ӧ����ȡ����Ӧ
+H2O����Ӧ����ȡ����Ӧ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��1��������β����ȼ�ղ��������������������������CH4����ԭNOx���������������Ⱦ��
��1��������β����ȼ�ղ��������������������������CH4����ԭNOx���������������Ⱦ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
��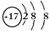 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
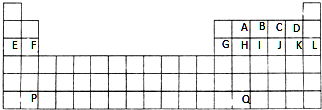
 ������Ԫ��X��Y��Z��W�����ڱ��е�λ����ͼ��ʾ������W�����������γ��������Ҫ����֮һ��
������Ԫ��X��Y��Z��W�����ڱ��е�λ����ͼ��ʾ������W�����������γ��������Ҫ����֮һ��| X | |||
| Z | W | Y |
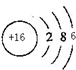 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��T����ͻ��ϼ�Ϊ-3����J����������ϼ�Ϊ+6 | |
| B�� | ��HnJOmΪǿ�ᣬ��G��λ�ڢ�A�Ժ�Ļ��÷ǽ���Ԫ�� | |
| C�� | ��M��OH��n+1Ϊǿ���R��OH��nҲΪǿ�� | |
| D�� | ��M����������ϼ�Ϊ+4��������Ԫ�ض��Ƿǽ���Ԫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NH4+��NO3-��K+��SO42- | B�� | CO32-��NO3-��HCO3-��Na+ | ||
| C�� | Na+��ClO-��AlO2-��NO3- | D�� | Cu2+��K+��Na+��SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
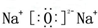 ��F��ԭ�ӽṹʾ��ͼΪ
��F��ԭ�ӽṹʾ��ͼΪ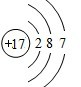 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ������и�һ��9�µ��л�ѧ�Ծ��������棩 ���ͣ�ѡ����
���й���ʵ���������������������ȷ����
A. ����������м�������ʱ������Ҫ���ʯ��������ֱ�ӽ��м���
B. ����ʵ���У�Ҫ����ƿ�м��뼸����ʯ�����Ƭ���Է�ֹҺ�巢���ֲ����ȶ�����
C. ����ʱ������ˮӦ���������Ͽڽ����¿ڳ�
D. ��Һʱ����Һ©���е��²�Һ����¿ڷų����ϲ�Һ����Ͽڵ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com