
| 300�� | K2O��N2 |
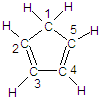 ��ͼ�����ֽ�����̼ԭ�ӱ�ţ�������5��̼ԭ���в�ȡsp3�ӻ�����
��ͼ�����ֽ�����̼ԭ�ӱ�ţ�������5��̼ԭ���в�ȡsp3�ӻ�����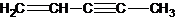 �ǻ�ï��ϩ��һ��ͬ���칹�壬������ӽṹ�д���ͬһƽ���ϵ�ԭ�Ӹ��������
�ǻ�ï��ϩ��һ��ͬ���칹�壬������ӽṹ�д���ͬһƽ���ϵ�ԭ�Ӹ��������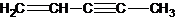 �д���ͬһƽ���ϵ�ԭ�Ӹ��������9�����ʴ�Ϊ��1��9��
�д���ͬһƽ���ϵ�ԭ�Ӹ��������9�����ʴ�Ϊ��1��9�� 2-���ʴ�Ϊ��C22-��
2-���ʴ�Ϊ��C22-��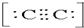 2-��
2-��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
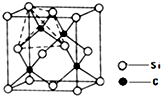 ��2013?�ൺһģ��[��ѧ-���ʽṹ������]
��2013?�ൺһģ��[��ѧ-���ʽṹ������]�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| I1 | I2 | I3 | I4 | �� |
| 496 | 4562 | 6912 | 9540 | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
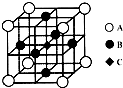 ��2013?��ͨһģ�������ʽṹ�����ʡ�
��2013?��ͨһģ�������ʽṹ�����ʡ�



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ������ | AF | BF2 | DF4 |
| �۵�/K | 1266 | 1534 | 183 |
| 1 |
| 2 |
| 3 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2011?֣�ݶ�ģ��[��ѧ--ѡ�����ʽṹ������]
��2011?֣�ݶ�ģ��[��ѧ--ѡ�����ʽṹ������]�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com