
ij��ѧ����С����ʵ�����������ͼ��ʾ��ʵ��װ�ã����С����Ĵ�������ʵ�顣

(1)A�������巢��װ�ã�A�����õ��Լ�ֻ�ܴ�����������ѡȡ��
������泥���̼��泥���̼����泥����Ȼ�泥�����ʯ�ң����������ơ�
��A����ȡ����ʱֻ����һ��ҩƷ�����ҩƷ������__________(��ѡ����)����ֻ��һ��ҩƷ��ȡ����ʱ��ͼ�пհ״���������ӦΪ____________(ѡ������������ţ��̶�װ��ʡ��)��


(2)��װ�ò�����������Ȼ����һ����ȱ�ݣ��ԴӰ�ȫ�뻷���ĽǶ������ǣ��Ը�װ�ý��иĽ���
��________________________________________________________________________��
��________________________________________________________________________��
(3)���ոĽ����װ�ý���ʵ�飬������������⣺
��װ��B��������_____________________________________________________��
��д��C�з�����Ӧ�Ļ�ѧ����ʽ��_______________________________________��
����A��B���Լ���������װ��D�п��Թ۲쵽��ʵ��������________________________________________________________________________��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������ЧӦ����Դ��ȱ�����⣬��ν��ʹ����е�CO2���������Կ������ã������˸������ձ����ӡ�Ŀǰ��ҵ����һ�ַ�������CO2����ȼ�ϼ״���CO2(g)��3H2(g)CH3OH(g)��H2O(g)���÷�Ӧ���й����������ı仯(��λΪkJ/mol)��ͼ��ʾ����ش��������⣺

(1)�۲�ͼ���֪������Ӧ�����У����ѷ�Ӧ���еĻ�ѧ�����յ�������________________(����ڡ�����С�ڡ����ڡ�)�γ��������еĻ�ѧ���ͷŵ���������
(2)�״��Ľṹ�������Ҵ�����д���״��Ľṹʽ��_________���״������еĻ�ѧ��������________________(����Ӽ������ۼ���)��
(3)������Ա������һ�����ͼ״�ȼ�ϵ�ء���������Һ��KOH��Һ���ڸõ�صĸ���������Ӧ��������________________����������________________(���������ԭ��)��Ӧ��
(4)��ʹ�ϳɼ״��Ļ�ѧ��Ӧ���ʼӿ죬��д��������ʩ��_____��
(5)������ʵ�ܱ����÷�Ӧ�Ѵ�ƽ��״̬����_______(�����)��
A��CO2(g)��Ũ�Ȳ��ٷ����仯
B����λʱ��������1 mol CO2��ͬʱ����3 mol H2
C���ڡ������ȵ������У��������¶Ȳ��ٷ����仯
D����һ���ݻ��̶��������ڣ�ѹǿ���ٷ����仯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��̬NH4Cl���ȱ�����壬���������ֱ�Ϊ��̬NH4Cl����̬�����ȱ�����������������ֱ�ɹ�̬�⣬����������ı����Ƿ���ͬ����˵���жϵ����ɡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵������ȷ���� (����)
A������ֲ��ͨ�����������տ����еĵ��������ڻ�ѧ�仯
B������β�����ŷŵĵ�����������Ҫ��������̬��ת������
C����ʯȼ��ȼ��ͨ�����ͷų�����������
D��ֲ��ո�ȼ��ʱ�ų���������������˵���ѭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ���� (����)
A����װ��Fe(NO3)2��Һ���Թ��м���ϡH2SO4���ڹܿڹ۲쵽����ɫ���壬���ۣ�HNO3�ֽ����NO2
B��ʵ���Ҽ��鰱���ķ����ǽ�ʪ�����ɫʯ����ֽ����ƿ�ڻ�ܿڣ��۲���ֽ�Ƿ��
��ɫ
C�����ȵIJ�˿��NH3��O2������Ӵ�����˿�������ֺ��ȣ�˵������������Ӧ�Ƿ��ȷ�Ӧ
D��Ũ������ǿ�����ԣ�����������Fe�������ҷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ������NaOH��Һ����μ���AlCl3��Һ�����ɳ���Al(OH)3������AlCl3�������ı仯��ϵ��ͼ��ʾ���������������ڶ�Ӧ����Һ��һ���ܴ����������(����)
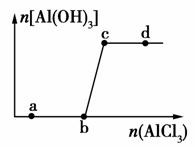
A��a���Ӧ����Һ�У�Na����Fe3����SO ��HCO
��HCO
B��b���Ӧ����Һ�У�Ag����Ca2����NO ��F��
��F��
C��c���Ӧ����Һ�У�Na����S2����SO ��Cl��
��Cl��
D��d���Ӧ����Һ�У�K����NH ��I����CO
��I����CO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й����ʵ�������Ӧ�������Ӧ����(����)
A�����Ľ���������ǿ���ʲ����ø�ƿ��ʢ��Һ��
B��Na2O2��CO2�ܷ�Ӧ��������������������ߺ�DZˮͧ������
C��NaHCO3�����ֽܷ����CO2���壬��ʳƷ��ҵ�п���Ϊ���Ƹ������ɼ�
D��������Ũ������ʹ�������ۻ��������¿�������������������Ũ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ����
A����֪2SO2��g��+ O2��g�� 2SO3 ��g�� ��H��0���÷�Ӧ���κ��¶��¾����Է�����
2SO3 ��g�� ��H��0���÷�Ӧ���κ��¶��¾����Է�����
B��0.01 mol��L-1NaHCO3��Һ��c��Na����= c��HCO3 -��+ 2c��CO32-��+ c��H2CO3��
C��25�棬0.1mol��L-1 K2CO3��Һ��c��H+��/ c��OH���� =l.0 �� l0-a������Һ��pH=7+0.5a
D�������£�Ksp��AgCl��=1.8��10-10��Ksp��Ag2CrO4��=9.0��10-12����Ũ����ȵ�Na2CrO4��NaCl�Ļ��ϡ��Һ�еμ�0.01 mol��L-1 AgNO3��Һ��������Ag2CrO4����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ڱ�״�����У���6.72 L CH4����3.01��1023��HCl���ӣ���13.6 g H2S����0.2 mol NH3�����ж������������������ȷ���� (����)
a������ڣ��ۣ��٣��� b���ܶȢڣ��ۣ��ܣ���
c�������ڣ��ۣ��٣��� d����ԭ�Ӹ����٣��ۣ��ܣ���
A��abc B��bcd C��abd D��abcd
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com